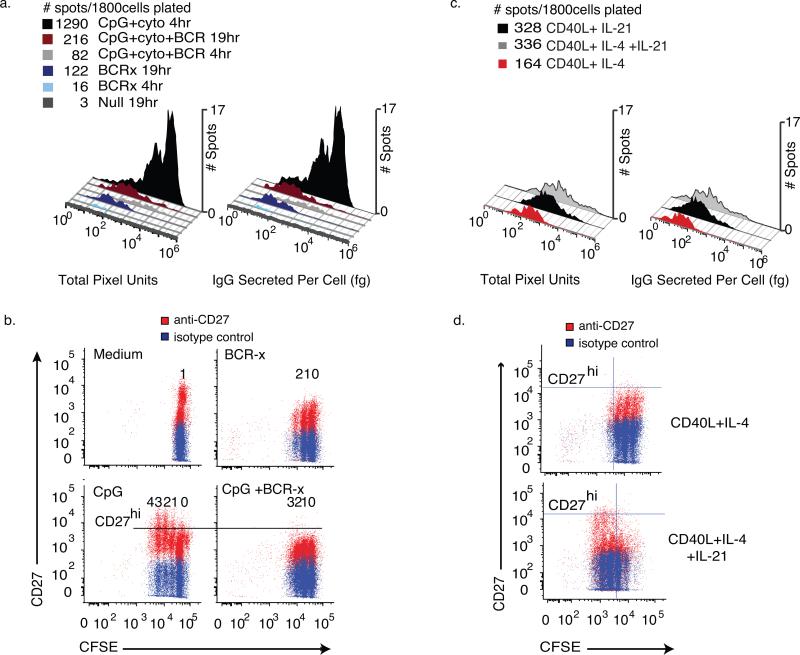
Figure 6. Estimating IgG release from individual cells in mixed populations of responding memory B cells.
Human CD27+ memory cells depleted of IgM+ B cells were isolated and split into groups cultured with medium, CpG2006 ODN and cytokines (CpG+ck) and BCR-crosslinking antibodies (BCR-x), alone or in combination. After 96 hours of culture, the cells were washed and assayed for IgG secretion in paired ELISA and ELISPOT assays next to wells pre-spotted with bead standards for IgG release. Cells were incubated in the assay plates for 4 hours or 19hours (N=6wells, 300 live cells/well). Using a bead-derived standard curve as in Fig. 4, values for IgG secreted were calculated. (a) Histograms of raw spot intensity and estimated IgG secretion. The addition of BCR-crosslinking antibodies to the CpG+CK stimulation conditions reduced the number of high- σIgG IgG-SC by 92.7 ± 4.73% and low range σIgG IgG-SC by 53.3 ± 18.7%. Spots on plates allowed to incubate 19hrs show low σIgG (IgG<1000 fg•cell−1) cell populations more clearly. (b) Flow cytometric analysis of IgMneg memory B cells stained with CFSE at isolation, stimulated with CpG+CK stained for viability and CD27 cell surface marker expression after 72 hours of incubation. Undivided cells are seen as the population furthest to the right, each division producing an additional population with reduced CFSE staining. With each generation, an increase in CD27high proliferating B cell blasts can be seen in CpG+CK-stimulated cells and the number of CD27high cells is reduced by the addition of BCR-x. (c) Spot Intensity and estimated IgG secreted per cell histograms of IgM-depleted CD19+CD27+ memory B cells that had been stimulated with CD40L-expressing fibroblasts and rhuIL-4 or rhuIL-21, alone or in combination for 96 hours, washed and assayed as in figure 6a. CD40L and IL-4 stimulation yielded low σIgG IgG-SC. Activation by CD40L+IL-21, with or without IL-4 induced a small population of high σIgG IgG-SC and increased total spot numbers. (d) CFSE vs CD27 expression of cells treated as in (c). In cell populations treated with IL-21, more cell division is evident and CD27 expression increases in dividing cells, consistent with development of a B cell blast phenotype.