Figure 4.
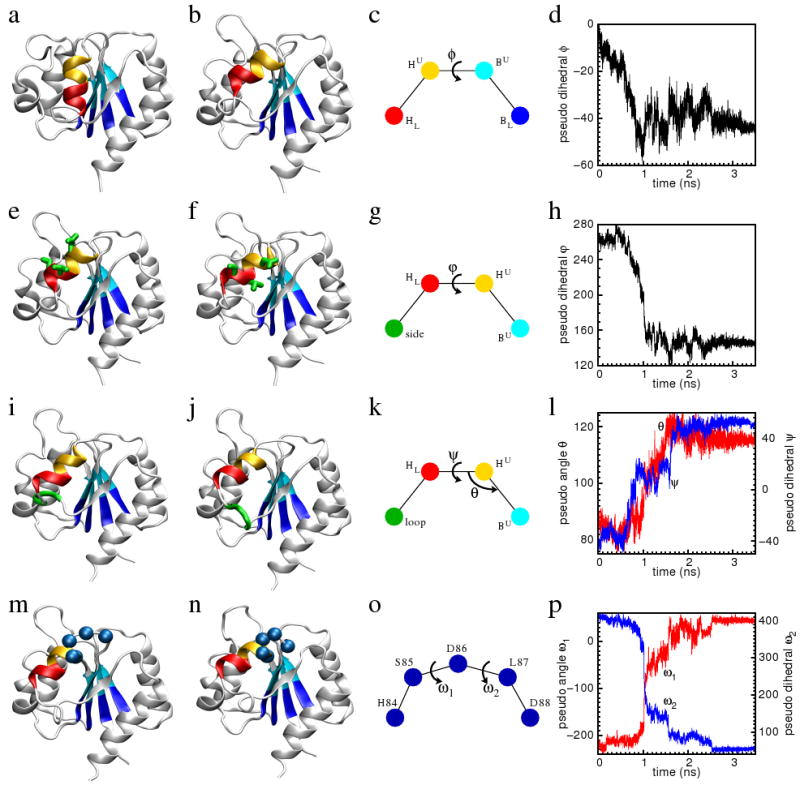
The characteristic motions and reaction coordinates of the tilt (the first row), the rotation (the second row), the flip (the third row), and the adjust (the fourth row) stages of the transition. In each row, the first two sub-figures are the conformations before and after the transition. The third sub-figure illustrates the reaction coordinates of the transition. The colored spheres represent the center of masses (CM) of the residues of the same color shown in the first two sub-figures of the same row. The reaction coordinates are designated by Greek letters. The fourth sub-figure shows the time traces of the reaction coordinates along the whole TMD trajectory. The residues in the lower half of the first four β-stands (residues Val6, Trp7, Cys30, Thr31, Val50, Leu51, Val78 and Ile79) are colored blue. Their CM is designated as BL. The residues in the upper half of the first four β-stands (residues Val8, Val9, Thr32, Phe33, Leu52, Ser53, Ile80 and Met81) are colored cyan. Their CM is designated as BU. The residues in the upper half of the α4-helix (residues Leu87, Asp88, Ala89, and Ala90) are colored orange. Their CM is designated as HU. The residues in the lower half of the α4-helix (residues Val91, Ser92, Ala93, and Tyr94) are colored red. Their CM is designated as HL. In Figure (e) and (f), residues Asp88, Val91 and Ser92 are rendered by thick sticks and colored green. Their CM is designated as side. In Figure (i) and (j), the three residues Gln96, Gly97 and Ala98 are rendered as thick ribbon and colored green. Their CM is designated as loop. In Figure (m) and (n), the Cα-atoms of residues His84, Ser85, Asp86, Leu87 and Asp88 are rendered as spheres and colored blue.
