Abstract
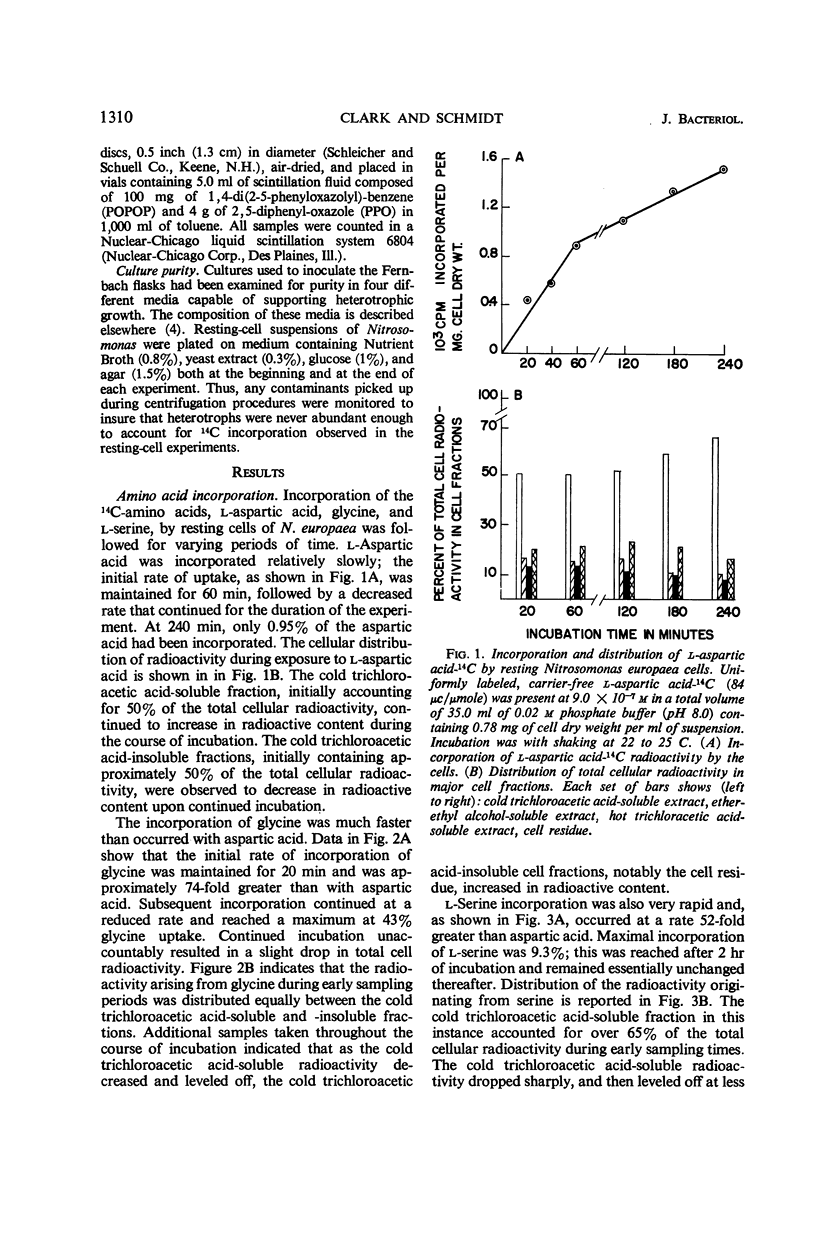
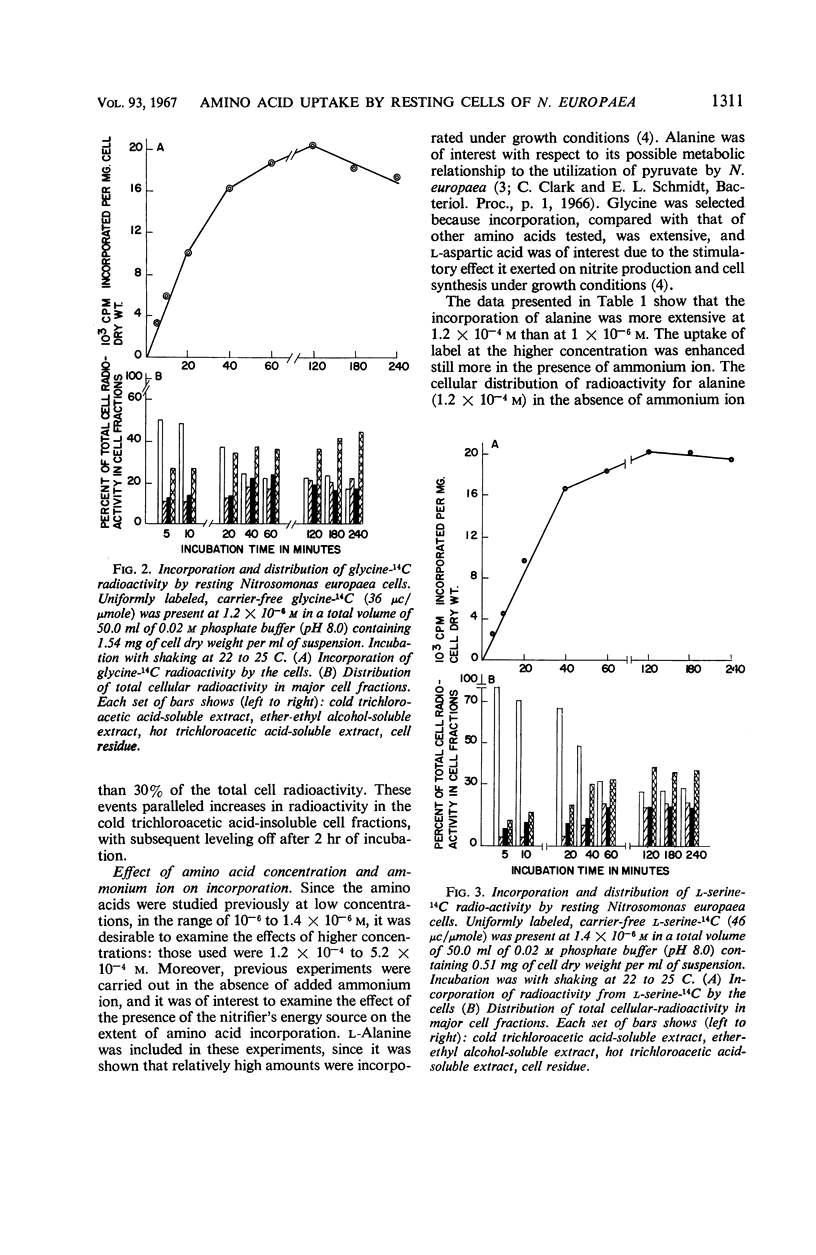
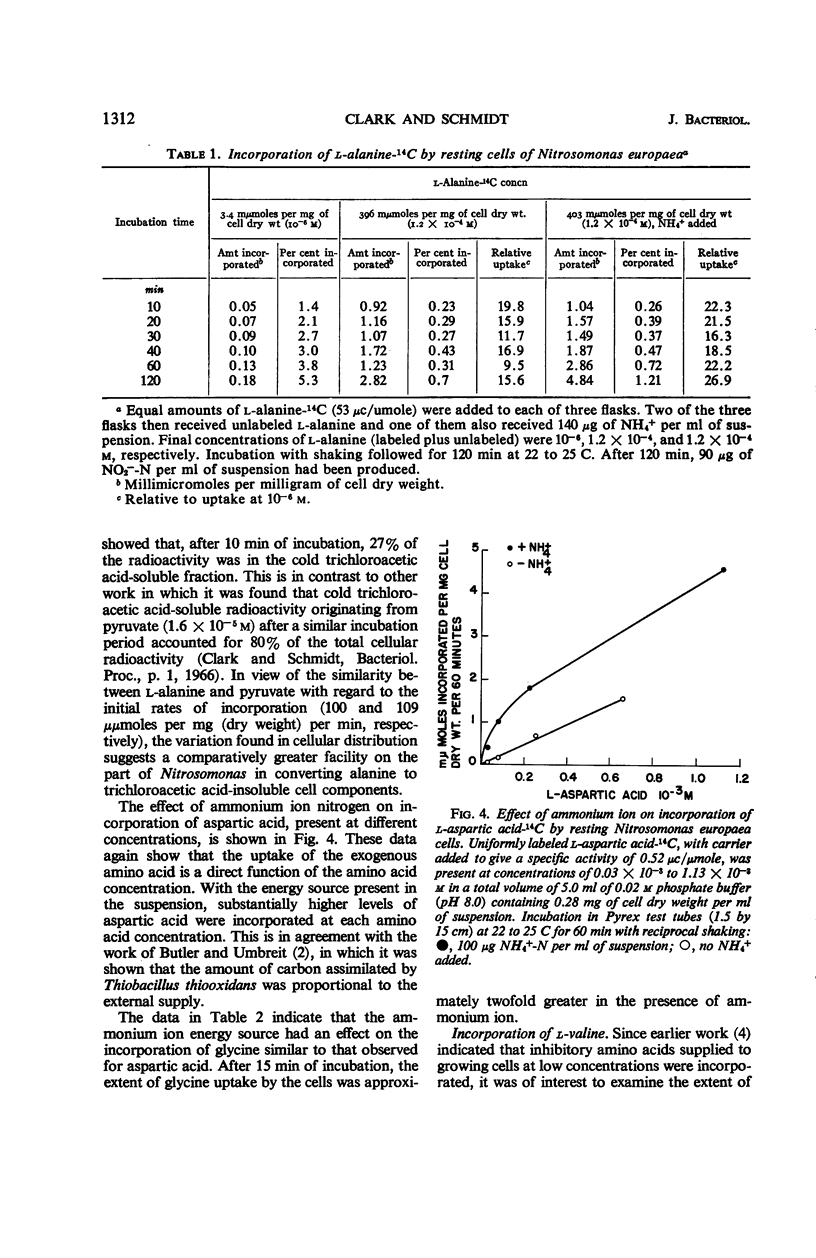
Incorporation of selected amino acids by resting cells was studied with regard to effects of concentration, rate and extent of incorporation, cellular distribution, effect of ammonium ion on uptake, and competitive effects. l-Aspartic acid, l-alanine, l-serine, and glycine presented at trace levels were incorporated at rates ranging from 0.11 to 8.2 μμmoles per mg (dry weight) per minute, and maximal incorporation was 11 to 333 μμmoles per mg (dry weight). When glycine and aspartic acid were supplied at substrate level, the rate of incorporation increased 14- and 109-fold, respectively. The presence of ammonium ion further increased both the rate and extent of uptake of glycine and aspartic acid. The distribution of cellular radioactivity arising from 14C amino acids indicated that cell pool radioactivity was concentrated from 1.2- to 24.5-fold over the external medium. Aspartic acid pool radioactivity accounted for 50% or more of the total cellular radioactivity, whereas radioactivity in glycine and serine pools dropped from initially high levels to 20 to 25% during incubation. The decrease in pool radioactivity with both glycine and serine was accompanied by an increase in other fractions, especially in the cell residue. The growth-inhibiting amino acid l-valine, supplied at substrate level, contributed more carbon per milligram (dry weight) than any other amino acid studied. l-Leucine, in the presence of l-valine, was observed to decrease valine incorporation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. G., Umbreit W. W. Absorption and utilization of organic matter by the strict autotroph, Thiobacillus thiooxidans, with special reference to aspartic acid. J Bacteriol. 1966 Feb;91(2):661–666. doi: 10.1128/jb.91.2.661-666.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Effect of mixed culture on Nitrosomonas europaea simulated by uptake and utilization of pyruvate. J Bacteriol. 1966 Jan;91(1):367–373. doi: 10.1128/jb.91.1.367-373.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Finstein M. S. Carbon and Energy Sources for the Nitrifying Autotroph Nitrobacter. J Bacteriol. 1965 Jul;90(1):102–107. doi: 10.1128/jb.90.1.102-107.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. O., COHEN G. N. Incorporation des amino-acides endogènes et exogènes dans les protéines de la levure. Ann Inst Pasteur (Paris) 1958 Jul;95(1):73–87. [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


