Abstract
Rationale
Butorphanol exerts activity at mu, kappa and delta opiate receptors in rats and monkeys but produces predominant mu-like effects in humans.
Objectives
To determine if the kappa receptor-mediated actions of butorphanol could be unmasked or enhanced by giving it in combination with naltrexone, an opioid antagonist with higher affinity for mu versus kappa receptors.
Methods
Ten healthy adult inpatient volunteers (8 M, 2 F), with opioid abuse histories, completed this double-blind, randomized, placebo-controlled study. Naltrexone (0, 1, 3, 10 or 30 mg, p.o.) was administered 1 hr before butorphanol (0, 6 or 12 mg/70 kg, i.m.) during 15 test sessions. An array of physiological (e.g., vital signs, urine output, subject- and observer-rated measures was collected before and for 4 hr after drug administration.
Results
Naltrexone alone produced no direct effects. Butorphanol alone produced typical mu-, but not kappa-, related physiological effects (e.g., miosis, respiratory depression) and produced mood and drug effects considered typical of both mu (e.g., “liking,” “good drug effects”) and kappa agonists (e.g., increases in perceptual disturbances). Naltrexone pretreatment led to significant butorphanol-induced diuresis (i.e., increased urine output and decreased urine osmolality). Naltrexone generally produced a dose-dependent blockade of these subjective responses.
Conclusion
These data suggest that naltrexone antagonism unveiled the kappaergic activity of butorphanol as measured by diuresis, while subjective responses generally attributed to mu versus kappa receptors were not dissociable. Moreover, these data demonstrate that butorphanol exerts physiologically relevant kappa agonist activity at these supraanalgesic doses in humans.
Butorphanol tartrate is a synthetic opioid that is marketed in the United States as an injectable formulation and as a metered nasal spray; it is used clinically as an analgesic and as a pre-anesthetic agent for its sedative properties (for reviews, see (Gillis et al. 1995; Heel et al. 1978; Pachter and Evens 1985). In vitro (Commiskey et al. 2005; Emmerson et al. 1996; Gharagozlou et al. 2002) and in vivo studies (Horan and Ho 1989; Leander 1983b; Pircio et al. 1976) have characterized butorphanol as having intermediate agonist activity at mu, kappa and delta opiate receptors. Butorphanol is controlled under Schedule IV of the Controlled Substances Act reflecting the general acceptance that it has a lower abuse liability than morphine and other full mu opioid agonists, although there have been reports of both diversion and abuse of butorphanol (for example, see (Loder 2006).
While both mu and kappa receptor agonists can produce a constellation of pharmacodynamic actions, some of which overlap (e.g., analgesia and sedation), mu and kappa receptor activation can produce distinctive physiological responses (e.g., mu receptor activation results in pupil constriction; kappa receptor activation results in diuresis) allowing for assessment of their relative activation in vivo. Studies have shown that the degree to which the mu versus kappa effects of butorphanol is expressed differs depending upon the relative receptor affinity, the test species, and the study preparation. One reliable physiological indicator of kappa receptor activation is the production of diuresis. Increased urinary output has been observed after administration of selective kappa agonists, such U50,488 (Vonvoigtlander et al. 1983), enadoline (CI-977)(Hunter et al. 1990; Leander et al. 1985), spiradoline (Rimoy et al. 1991), and ethylketocyclazocine (Slizgi and Ludens 1982). The findings with butorphanol on urinary outcomes have been mixed. For example, Leander (1983) reported that butorphanol produced diuresis in mice but with less than half of the maximal effect produced by bremazocine (Leander 1983a); this is consistent with its characterization as a partial kappa agonist. Studies in rats have reported butorphanol-induced diuresis in normally hydrated rats (Craft and McNiel 2003; Horan and Ho 1989), and in female, but not male, rats under water-loaded conditions (Craft et al. 2000). In contrast, no significant diuretic effects were produced by butorphanol over a broad range of acute doses when tested in the rhesus monkey (Butelman et al. 1995). However, when clocinnamox, an irreversible mu antagonist was used as a pretreatment to block mu-mediated actions, butorphanol produced significant diuresis in rhesus monkeys that persisted even as much as two weeks after the antagonist treatment (Vivian et al. 1999). A review of the literature revealed no published studies on butorphanol-induced diuresis in humans, and, given its widespread clinical use in medical settings, the absence of reports suggest that this is not recognized as a problematic side effect of butorphanol.
Studies characterizing the subjective profile of action with butorphanol in humans have also yielded mixed results with respect to the relative prominence of the mu versus kappa effects. A series of well-controlled drug discrimination studies in human subjects conducted by Preston and colleagues characterized the discriminative stimulus effects of butorphanol under an array of conditions. In the first study, sporadic opioid abusers were trained in a three-way procedure to discriminate placebo, pentazocine (a mixed mu/kappa agonist reported to have greater kappa than mu activity), and hydromorphone (a full mu agonist; (Preston et al. 1989). When butorphanol (up to 6 mg/70 kg, i.m.) was substituted for the training drug, responses indicated that the butorphanol discriminative stimulus was most like pentazocine. However, when butorphanol was evaluated in a two-way discrimination in subjects trained to discriminate hydromorphone from placebo, butorphanol (up to 6 mg/70 kg, i.m.) showed generalization to hydromorphone, consistent with its known mu agonist activity (Preston et al. 1992). A later study followed up on this finding and trained subjects to perform a hydromorphone versus “not hydromorphone” discrimination; with those instructions, butorphanol (up to 6 mg/70 kg, i.m.) did not generalize to hydromorphone (Preston and Bigelow 2000). Finally, when butorphanol was examined in opioid dependent volunteers (i.e., maintained on 30 mg methadone/day) trained to discriminate placebo, hydromorphone and naloxone, butorphanol, at doses as low as 1.05mg/70 kg, substituted for naloxone – likely due to its ability to precipitate withdrawal as a result of its mu partial agonist properties (Preston et al. 1990). Thus, this array of studies suggested that butorphanol can act like a mixed kappa/mu agonist, a selective mu agonist or even a mu antagonist depending upon the test conditions.
When directly examined under acute dosing conditions on subjective effect measures, butorphanol produces a profile of effects that is different from that produced by either a mu agonist or kappa agonist. Zacny and colleagues examined acute doses of butorphanol (0, 0.5, 1 & 2 mg/70 kg, i.m.) in healthy volunteers (i.e., without opioid abuse histories) in comparison to those produced by morphine (10 mg/70 kg morphine, i.m.) (Zacny et al. 1994). They noted that, while there were some subjective responses common to both drugs (e.g., increased ratings of sedation and high), butorphanol produced a significant endorsement of subjective effects that was not noted for morphine (e.g., difficulty concentrating, dreaming, floating and sweating). These are signs and symptoms that have typically been included in opioid agonist/antagonist questionnaires and differentiate selective mu agents from agents with mixed receptor actions (Fraser et al. 1961). It was also noted that butorphanol, but not morphine, produced significant performance impairment on psychomotor tasks, including the digit-symbol substitution task and an eye-hand coordination task. Acute doses of butorphanol over a higher dose range (0, 3 & 6 mg/70 kg, i.m.) were also examined in experienced, but not physically dependent, heroin abusers in comparison to morphine (15 & 30/70 kg, i.m.) and lorazepam (4 mg/70 kg, i.m.) (Greenwald and Stitzer 1998). Again, while butorphanol shared some global effects with morphine, butorphanol produced other direct effects that were not common to morphine but were, in some cases, common to lorazepam (e.g., dysphoria, confusion and increased scores on the PCAG scale of the ARCI (Martin et al. 1971). Finally, we compared the pharmacodynamic actions of butorphanol (0, 1.5, 3, 6 & 12 mg/70 kg, i.m.) to both the selective kappa agonist, enadoline (20, 40 80 & 160 μg/70 kg, i.m.), and to the full mu agonist, hydromorphone (Walsh et al. 2001b). In this case, the profile of subjective effects for butorphanol overlapped considerably with hydromorphone (e.g., increased ratings of good effects, liking, nodding, etc.), but shared few non-mu symptoms with enadoline (i.e., increased ratings of confusion). Interestingly, all three drugs overlapped in their ability to increase global ratings of “high” and observer ratings of “coasting/spaced out.” As in other studies, butorphanol produced miosis and mild respiratory depression like hydromorphone. Conversely, enadoline produced significant diuresis, while butorphanol led to mild urinary retention in comparison to the placebo control condition. These findings suggested that, when given in acute doses to humans, the mu-mediated actions of butorphanol predominate over the kappa-like effects for both subjective and physiological responses.
We were initially interested in butorphanol because of its potential kappa activity and the recognized potential of kappa agents for use in the treatment of cocaine dependence (see (Walsh et al. 2001b). However, there are numerous unwanted side effects after administration of selective kappa agonists, including diuresis, sweating, sedation, dizziness, visual distortions, feelings of depersonalization, dysphoria, and, at higher doses, psychotomimetic effects (Dershwitz et al. 1991; Kumor et al. 1986; Walsh et al. 2001b). This host of undesirable effects would likely limit their utility as pharmacotherapies, and indeed limited our ability to test higher doses of the full kappa agonist, enadoline, due to problematic dysphoric and hallucinogenic-like effects (Walsh et al. 2001b). We identified butorphanol as a potential agent of interest speculating that its combined partial kappa agonist and partial mu agonist effects could make it more readily tolerated and reinforcing, respectively, thus enhancing its therapeutic acceptability. However, because the predominant profile of actions was mu-like when compared directly to a selective kappa agonist (Walsh et al. 2001b) and it was ineffective in altering cocaine self-administration (Walsh et al. 2001a), it was unclear whether butorphanol had sufficient in vivo kappa activity to test the hypothesis. Therefore, we were interested in determining whether the kappa receptor-mediated actions of butorphanol could be unmasked or enhanced by giving it in combination with an opioid antagonist to reduce or block its predominant mu-mediated effects. Because data from human studies have suggested that the dysphoric subjective effects of butorphanol become more prominent when higher doses are administered (e.g., (Houde et al. 1976; Jasinski et al. 1975), a higher dose range of butorphanol was tested to ensure adequate kappa activity. Naltrexone, which displays approximately a 10-fold greater affinity for mu compared to kappa receptors (Ko et al. 1998) was used as a pretreatment over a range of doses known to produce varying degrees of mu blockade in humans. Thus, the purpose of this study was to evaluate the relative mu versus kappa receptor-mediated pharmacodynamic actions of butorphanol under conditions of differential naltrexone receptor blockade over a range of doses in experienced, but not physically dependent, opioid abusers.
METHODS
Subjects
Participants were twelve healthy adult volunteers who were opioid-experienced but not physically dependent on opioids at the time of the study. Volunteers were recruited through local newspaper advertisements and word of mouth, and were paid for their participation. Before study entry, subjects received complete physical examinations, which included ECG and blood and urine chemistries. All subjects reported regular abuse of illicit drugs other than heroin, with cocaine being the most frequently reported secondary drug of abuse. Study enrollment was continuous, and a maximum of four volunteers could participate simultaneously.
All participants were determined to be in good health by physical examination, an electrocardiogram, laboratory tests, and medical history. They were without significant psychiatric disturbance other than their drug use according to a structured psychiatric interview (Structured Clinical Interview for the DSM-IV; (First et al. 1996)). Participants were not physically dependent on opioids at the time of enrollment as determined by self-report as well as objective evidence, including residential observation while drug-free and at least one urine test negative for opioids during the recruiting and intake process. This study was approved by the Johns Hopkins Bayview Medical Center Institutional Review Board; subjects gave their written informed consent prior to participation. This study was conducted in accordance with the Helsinki guidelines for ethical human research.
Subjects resided on a closed 14-bed residential research facility for approximately 8 weeks while participating in the study. This unit is staffed by licensed nursing personnel 24 hr/day and is used exclusively for behavioral pharmacology research. Recreational activities, exercise equipment, arts and crafts projects, television and video games were available. Urine specimens were randomly obtained and tested for illicit drugs to ensure the absence of drugs other than those administered experimentally. Breathalyzer tests were given on admission and on a random basis for the same purpose. There were no positive drug or alcohol screens found during these random drug tests. Subjects were maintained on a caffeine-free diet. Nine of the ten subjects were smokers and were allowed free access to cigarettes, except during experimental sessions. Subjects were given a light breakfast (e.g., cereal, toast, juice) 2 hrs prior to the experimental sessions.
Drugs
Butorphanol tartrate (Apothecon, Princeton, NJ) was aseptically prepared under a horizontal laminar flow hood by filtering the solution through a 0.22-mm Millex-GS Millipore filter (Millipore Products Division, Bedford, MA) into a sterile, pyrogen-free vial (Lyphomed, division of Fujisawa USA, Inc., Deerfield, IL). Butorphanol doses were prepared from a pre-formulated stock solution (Stadol© 2 mg/ml) by diluting to the correct volume with saline. Butorphanol was administered by intramuscular injection. All doses were based on admission body weight and were calculated in mg of the salt weight per 70 kg body weight. All doses were formulated for administration in a volume of 8 ml (the smallest possible volume given the concentration of the stock solution). This volume was administered in three divided injections to avoid tissue damage, two 3-ml injections to the right and left glutei maximi and one 2-ml injection to the right or left deltoid. Placebo consisted of the same volume of sterile saline for injection.
Naltrexone was obtained from a commercial source in tablet formulation (Du Pont Company, Wilmington, DE). Ten 50-mg tablets were weighed and then pulverized with a mortar and pestle. The amount of powder equivalent to each dose was calculated and weighed separately for each volunteer. The powder was placed in an opaque capsule and filled with lactose USP (Malinckrodt Chemical, Inc., St. Louis, MO). Matched placebos consisted of identical capsules filled with lactose.
Rationale for Dose Selection
In the absence of binding studies from humans, the rationale for dose selection in the present study was informed largely by published binding affinities from preclinical studies, imaging studies, and the extant literature on the clinical pharmacodynamics of these drugs. Binding studies suggest that naltrexone displays preferential binding for mu over kappa receptors ranging from 3-fold (Ananthan et al. 1999) to 8-fold (Martin, 1984). Perhaps the most relevant findings are those from in vivo analgesia data from rhesus monkeys employing a pA2 analysis, which suggested that naltrexone has an approximate 10-fold greater affinity for mu over kappa receptors (Ko et al. 1998). Naltrexone is typically prescribed for the treatment of opioid dependence at a dose of 50 mg/day. An earlier study used positron emission tomography to model and estimate the mu receptor occupancy in healthy human volunteers in response to naltrexone administration (Lee et al. 1988). Binding of [11C]-labeled carfentanil, a selective mu opioid agonist, was examined after treatment with a single dose of 50 mg oral naltrexone and maximum blockade was achieved at 1 hr, consistent. At 48 hrs post-dosing, the blockade remained at about 90% after this single dose, leading the authors to conclude that doses lower than 50 mg should lead to complete occupancy of opiate receptors. Observations from our laboratory confirm that nearly complete blockade of opioid agonist effects can be achieved with doses of naltrexone lower than 50 mg (Walsh et al. 1996). Thus, the range of naltrexone doses (1 to 30 mg) for this study were chosen with the aim of producing a broad range of mu opioid blockade in the context of no or very limited kappa receptor blockade (assuming an approximate 10-fold difference or less in vivo). Butorphanol is known to have binding affinity for the mu, kappa, and delta opioid receptors (Emmerson et al. 1996; Gharagozlou et al. 2002; Zhu et al. 1997). Data from rhesus monkeys, the species for which the pharmacodynamic effects of butorphanol are most concordant with humans, indicate that butorphanol exhibits an approximate 1:12 selectivity for displacement of ligands from mu versus kappa receptors (Ki = 0.5 to 6.1 nM, respectively; (Commiskey et al. 2005; Vivian et al. 1999) but see also Commiskey et al. 2005).
Study Design
A double-blind, randomized, placebo-controlled design was used. The first session served as a practice session and safety evaluation during which subjects received an active dose of butorphanol (6 mg/70 kg butorphanol). This served to acclimate the volunteer to the experimental environment, to screen for idiosyncratic reactions to butorphanol, and to confirm that the volunteers were able to provide subjective reports of opiate drug effects. Subjects participated in fifteen experimental test sessions in which they received a single dose of naltrexone (0, 1, 3, 10 or 30 mg, p.o.) 60 min before receiving an intramuscular dose of butorphanol (0, 6 or 12 mg/70 kg, i.m.); each naltrexone and butorphanol dose combination was tested once. Sessions were separated by at least 72 hrs. Baseline data were collected for 30 min prior to administration of the oral medication at 9:30 AM; the intramuscular drug was administered 1 hr later at 10:30 AM, and data collection proceeded for 4 hr thereafter.
Experimental Sessions
Subjects were escorted to the experimental test room at approximately 9:00 am and were seated in a cushioned chair directly in front of an Apple IIGS personal computer (Apple Computer, Cupertino, CA) used to collect the data. The computer was programmed to record physiological measures (except pupil diameter) and to present questionnaires in the appropriate order; volunteers entered their responses by using a computer mouse and/or keypad. A research assistant was seated behind the computer with a keyboard to initiate tasks and to enter observer-rated measures. Data printouts were collected after the completion of each session, and data were transferred to Macintosh Excel spreadsheets (Microsoft Corp., Redmond, WA) for analyses.
Physiological Measures
Volunteers were monitored continuously on a minute-by-minute basis throughout each session on a number of physiological variables. Oxygen saturation, skin temperature, blood pressure and heart rate were collected using an automatic physiologic monitoring device (Noninvasive Patient Monitor model 506, Criticare Systems, Waukesha, WI) and galvanic skin response was collected using an AT64 Portable SCR (Autogenics, Wood Dale, IL). An observer recorded the respiration rate by counting the number of breaths taken by the subject for a 15-sec period. Pupil diameter was determined from photographs taken in constant room lighting with a Polaroid camera (Polaroid Corp., Cambridge, MA) using a two-fold magnification. Data collection began 30 min before the oral drug administration, and continued for 4 hr after the intramuscular drug administration (beginning at 0900 hours and ending at 1430 hours).
Urine was collected throughout each experimental session in order to measure urine output (i.e., volume) and urine osmolality. Participants were instructed to void just prior to the capsule administration, and this sample was discarded. Subjects were given two 500 mL bottles of sodium-free water and were instructed to consume one bottle each during the first and second 2-hr periods after the intramuscular drug administration; this was monitored to ensure compliance. Urine (total void) was collected at the end of each 2-hr period (i.e., after hours 1 & 2 and hours 3 & 4). The total volume of the urine output was measured and recorded. Aliquots (5 ml) from each of these two voids were subsequently analyzed for osmolality.
Subject and Observer-rated Measures
Subject-rated measurements included visual analog scales, the Addiction Research Center Inventory (ARCI) short form (Martin et al. 1971), the Perception scale (Isbell et al. 1956), and a pharmacological class questionnaire (from (Jasinski et al. 1977)). An observer-rated questionnaire was also used. The entire battery was collected at 30 min before and after the capsule, and at 15, 30, 45, 60, 90, 120, 150, 180 and 210 minutes after the injection (along with pupil photographs and manual respiratory rate measurements).
The visual analog questions were “Do you feel any DRUG EFFECT?” “ Do you LIKE the drug?” “How HIGH are you?” “Does the drug have any GOOD EFFECTS?” “Does the drug have any BAD EFFECTS?” “Do you feel SEDATED?” and “Are you SEEING or HEARING things?” The subjects responded by positioning an arrow along a 100-point line labeled with “none” at one end and “extremely” at the other. The ARCI consisted of 49 true/false questions that are subdivided into scales that are sensitive to euphoria (morphine-benzedrine group: MBG), sedation (pentobarbital-chlorpromazine-alcohol group: PCAG), dysphoria (lysergic acid diethylamide: LSD), and amphetamine-like effects (benzedrine group: BG and amphetamine: A). The Perception scale consisted of 40 questions scored on a scale from 0–9. These items are subdivided into ten subscales representing the senses or domains, as follows: detachment, visual, auditory, taste, tactile, smell, dizziness, cognitive, paranoia and general scales. The original questionnaire was developed to detect the effects of LSD (Abramson et al. 1955); it was revised and expanded to the current version (Isbell et al. 1956) and has been further described by others (Kumor et al. 1986). On the pharmacological class questionnaire, subjects were asked to categorize their drug effect as being most similar to one of the following ten classes of psychoactive drugs: placebo, opiates, phenothiazines, barbiturates and sleeping medications, opiate antagonists, antidepressants, hallucinogens, benzodiazepines, stimulants and phencyclidine. Descriptive titles and examples were given for each class of drug.
Observers rated the volunteers on a scale from 0 (“not at all”) to 4 (“extremely”) on each of the following items: flushing, skin itchy, sweating, turning of stomach, nodding, relaxed, coasting or spaced out, and pleasant sick.
Statistical Analyses
The effects of naltrexone alone were examined by analyzing data collected from sessions during which intramuscular placebo was given (i.e., no active butorphanol) in combination with each dose of naltrexone (0, 1, 3, 10, 30 mg). All measures collected during the experimental sessions were analyzed initially as raw time course data using a three-factor analysis of variance (ANOVA: naltrexone × butorphanol × time). The on-line physiological measures, collected on a minute-by-minute basis during the experimental sessions, were averaged across time to yield intervals (ranging from 15 min to 30 min), which corresponded to the subjective reporting intervals. Urine samples were collected over two time periods; these were analyzed singularly (i.e., Time 1 and Time 2) and summed for Total Urine Volume. Osmolality was also analyzed as Time 1 and Time 2; these values were also averaged to yield the Mean Osmolality. In addition to the raw score analyses, peak scores (either minimum or maximum depending upon the direction of effects) for individuals were obtained for repeated measures collected during the experimental sessions; these were analyzed using 2-factor ANOVA (naltrexone × butorphanol). Tukey’s post-hoc tests for repeated measures were used to analyze further the time course and provide information on the time of onset and offset of effects across drugs and doses.
RESULTS
Subjects
Twelve volunteers were enrolled, and ten completed the study. Two subjects failed to complete the protocol; one subject was discharged due to reasons unrelated to the study, and one experienced an adverse reaction (described below). Data are presented from the ten subjects who completed the study. This group was composed of eight males and two females with an average age of 41.2 years; one volunteer was Caucasian and the remainder African-American. All ten subjects experienced opioid agonist effects during the first practice session in which 6 mg of butorphanol was administered. Volunteers reported using heroin an average of 10.4 days of the last 30 (range 5 – 18 days; median 8 days) with an average history of heroin use of 14.5 years. All of the volunteers reported abuse of other substances, including cocaine. None of the volunteers met criteria for physical dependence on any substance except for nicotine, and none met diagnostic criteria for current abuse or dependence of sedative/hypnotics, stimulants or hallucinogens.
Pharmacodynamic Effects of Naltrexone Alone
The effects of naltrexone alone were examined by culling those sessions during which placebo butorphanol was administered with each of the five naltrexone pretreatment doses. Naltrexone produced no significant effects on any scale from the visual analog, ARCI or Perception questionnaires. Similarly, naltrexone produced no significant direct effects on oxygen saturation, heart rate, skin temperature, blood pressure, pupil diameter or measures of galvanic skin response (p>.05). Naltrexone alone produced no direct effect on any measure of urine output or osmolality.
Pharmacodynamic Effects of Butorphanol Alone
Data shown in Figure 1 illustrate the time-action curve for butorphanol alone (from sessions when the naltrexone pretreatment was placebo) across an array of representative measures.
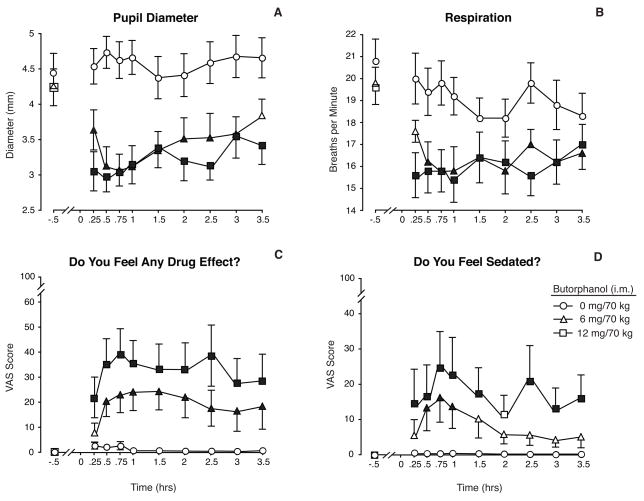
Figure 1.
Data shown illustrate the direct effects of butorphanol (0, 6 & 12 mg/70 kg, i.m.) when given after pretreatment with oral placebo. The baseline time point represents data collected 30 min prior to the injection. Measures shown include (a) pupil diameter, (b) respiratory rate, (c) visual analog data for the questions, “Do You Feel Any Drug Effect?” and (d) “Do You Feel Sedated?” All data shown are means (n=10) ± 1 S.E.M. Significant effects of butorphanol dose were found for all four measures (F [2, 18] = 41.4 pupil diameter; 20.3 respiratory rate; 12.3 drug effect, 3.5, sedated, 7.8; p ≤ .05). Significant main effects of time were also found for each measure (p ≤ .006). Filled symbols indicate a statistically significant difference (p < .05) compared to the baseline time point for that respective condition according to Tukey tests.
Physiologic Measures
The miotic effects of butorphanol are shown in Fig. 1A and illustrate that the effects on pupillary constriction were time-, but not dose-dependent, over the range of doses tested (statistical outcomes are provided in the legend). Significant miosis was evident within 15 min of injection, and these early effects were close in magnitude to the maximum effect produced. Pupils remained significantly constricted for the remainder of the experimental session. A similar profile of effects was observed for respiratory depression, whereby near maximal effects were achieved within 15 minutes of the injections; the magnitude of respiratory reduction was comparable for the 6 and 12 mg doses (Fig. 1B). Oxygen saturation was also reduced by butorphanol (F [2,18] = 27.3; p<0. 001); maximum reductions were approximately 1% on average (from 98–97%; data not shown), and effects were more pronounced for the higher butorphanol dose. Butorphanol alone produced significant increases in both systolic (F [2,18]=16.6; p<0. 001) and diastolic blood pressures (F [2,18]=12.5; p=0.001) in comparison to placebo. Overall, both butorphanol doses (6 & 12 mg) produced comparable increases and resulted in an average peak increase of approximately 10 mm Hg or less for both measures compared to baseline pressures. Similarly, butorphanol increased skin temperature significantly, and the magnitude of the response was nearly identical for the twobutorphanol doses (F [2,18]=13.2; p= 0.003). There was no main effect of butorphanol alone on heart rate, galvanic skin response or any measure of urine output; however, Tukey tests did reveal dose differences between 0 and 6 mg butorphanol on urine osmolality in the first 2 hours..
Subjective measures
Butorphanol produced a broad array of direct effects on subject-rated measures. Results from for the visual analog questionnaire, “Do you feel any drug effect?” (Fig. 1C) illustrate the general magnitude of effects reported by the subjects; there was a rapid onset of effects within 15 minutes of the injection and the peak effect was nearly achieved within the first half-hour. Effects were generally sustained for the remainder of the 3.5-hour test session and showed only modest signs of decline. Similar patterns of responding were observed for the visual analog scale ratings of “Liking,” “High,” and “Good Effects” (see Table 1 for statistical outcomes for all subjective and observer-rated measures). Ratings of “Do you feel sedated?” were significantly elevated by butorphanol; these ratings peaked at .75 hr and showed a general decline thereafter (Fig. 1D). There were no significant effects on the visual analog scale ratings for “bad effects” or ratings of “seeing or hearing things.”
Table 1.
Statistical outcomes for the analyses of subjective, observer-rated and physiological measures are shown. Values shown are p values, and only those measures for which significant outcomes were found are listed. 1, 2
| Butorphanol (df=2, 18) | Butorphanol × Naltrexone (df=8, 72) | |
|---|---|---|
| Visual Analog Scales2 | ||
| Any Effect | 0.0033 | 0.011 |
| Liking | 0.0063 | |
| High | 0.0033 | 0.0133 |
| Good Effects | 0.0103 | |
| Sedated | 0.0133 | 0.048 |
| Addiction Research Center Inventory | ||
| PCAG | 0.0123 | |
| BENZ | 0.044 | |
| LSD | 0.0033 | |
| SED | 0.0023 | |
| Perception Scale | ||
| General | 0.0233 | |
| Detachment | 0.0233 | 0.039 (peak only) |
| Cognitive | 0.0353 | |
| Visual | 0.038 (peak only) | |
| Taste | 0.044 (peak only) | |
| Total Perception Scale | 0.0313 | |
| Observer-Rated Adjectives | ||
| Nodding | 0.001 | |
| Coasting/Spaced Out | 0.012 | 0.023 |
| Physiological Measures | ||
| Pupil Diameter | 0.0013 | 0.0103 |
| Respiratory Rate | 0.0003 | |
| Oxygen Saturation | 0.0003 | |
| Skin Temperature | 0.003 | |
The original analysis was a 3-factor ANOVA (butorphanol dose × naltrexone dose × time). The main effect of naltrexone is not listed here, because those data were analyzed separately under the placebo butorphanol conditions as described in the text, and naltrexone failed to produce significant effects. The main effects of time and interactions with time are not shown either because they were so abundant and largely appeared whenever there were main effects of butorphanol or interaction effects between butorphanol and naltrexone.
Some measures already shown in the figures and described in the figure legends are not included in this table.
Indicates a significant main or interaction effect from the appropriate peak score analysis (peak minimum for pupil diameter, O2saturation, and respiratory rate and peak maximum for all others). This is in addition to the significant findings for the time course analysis.
ARCI
The time course analysis revealed significant main effects for butorphanol on the ARCI subscales, except for the Amphetamine and MBG scales. Scores on the PCAG and LSD scales were significantly increased, and scores on the Benzedrine scale were significantly decreased, by butorphanol in a time-, but not always dose-, related manner (statistical outcomes shown in Table 1). Peak ARCI scores on the PCAG andLSD scales occurred at 90 to 180 min after butorphanol injection, and were significantly increased at the p<0.01 level. These remained altered for the duration of the test session. Degree of sedation was not dose-related on the PCAG Scale.
Perception Scale
Scores on the General Perception, Detachment, Cognitive and Total Perception scales were significantly increased by butorphanol (Table 1). These were all dose-related effects and peaked early at about 30 minutes post-injection. For example, mean peak scores for the Detachment Scale were 0.5, 6.1, and 9.7, and for the Total Perception Scale were 2, 19.4 and 27 for 0, 6 and 12 mg/70 kg, respectively (p<.05). These responses were of shorter duration than those for the visual analog scales and tended to return to baseline by the end of the session. There were no significant main or interaction effects for butorphanol on the subscales for Auditory, Taste, Visual, Smell, Paranoia, Tactile or Dizziness scales.
Observer Measures
Butorphanol produced few observable signs. Significant, but modest, increases were seen on observer ratings of Nodding and Coasting/Spaced Out; these results were driven by the higher butorphanol dose as the low dose produced effects similar to placebo for these measures.
Interaction Between Naltrexone Pretreatment and Butorphanol
Physiologic Measures
The overall magnitude of effects seen on urine output and osmolality were more pronounced during Hours 1–2 after drug administration compared to those collected during Hours 3–4. By examining the outcomes when butorphanol was given in combination with placebo naltrexone, there were no significant direct effects of butorphanol (6 or 12 mg) in comparison to placebo on any measure of urine volume. However, butorphanol at 6 mg, but not 12 mg, given in combination with placebo naltrexone significantly reduced urine osmolality compared to the placebo butorphanol/placebo naltrexone condition during the first 2-hr collection period (Tukey test; p<.05; see Figure 2).
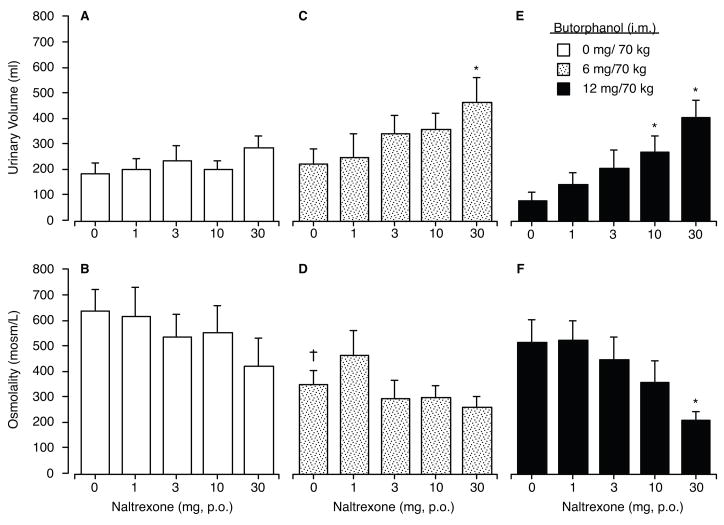
Figure 2.
Data shown in this figure are the mean values (n=10) for urine output during Hours 1 & 2 (Panels a, c & e) and urine osmolality assayed from that same time period (Panels b, d & f) after intramuscular dosing with placebo (left column), butorphanol 6 mg/70 kg (middle column) and butorphanol 12 mg/70 kg (right column) after 1-hr pretreatment with each of the five oral naltrexone doses shown along the abscissa. There was a main effect of naltrexone dose on urine volume (F [4,36] = 10.94; p<. 001) and urine osmolality (F [4,36]=5.56; p=. 001). Asterisks indicate significant differences from the naltrexone placebo control for that specific butorphanol dose (i.e., within the dose-effect function), crosses indicate a significant difference from the matched naltrexone dose condition when placebo butorphanol was administered, and vertical bars represent 1 S.E.M.
In contrast to the relative absence of direct effects of butorphanol, there were significant main effects of naltrexone when given in combination with butorphanol for nearly all measures of urine output. Data shown in Figure 2 illustrate the measures for urine volume (upper panels) and osmolality (lower panels) for the first 2-hr after butorphanol administration for each of the 15 dosing conditions. Data shown in the Fig. 2a reveal that naltrexone alone (i.e., in combination with placebo butorphanol) did not significantly alter urine volume output or urine osmolality (Fig. 2b). When butorphanol 6 mg/70 kg was administered (Fig. 2c), naltrexone produced a dose-dependent increase in urine output; however, only the 30 mg naltrexone dose was significantly greater than placebo (p<. 05; Tukey test). While the profile of effects after 12 mg/70 kg butorphanol was generally similar to that seen after 6 mg/70 kg butorphanol, the pattern was more orderly. Naltrexone produced significant and dose-dependent increases in urine output (Fig. 2e) along with a significant decline in urine osmolality (Fig. 2f).
The upper panel of Figure 3 illustrates the interaction between naltrexone pretreatment with butorphanol across the full range of test conditions on three physiological outcomes. Data shown in the first panel illustrate the effects on pupil diameter for which there were significant interaction effects between butorphanol and naltrexone according to both the time course and peak minimum score analysis. The data illustrate that naltrexone produced a dose-dependent blockade of the miotic effects of butorphanol. While the highest doses of naltrexone (10 and 30 mg) produced nearly complete blockade of the butorphanol 6 mg dose, the miosis produced by butorphanol 12 mg was not as completely blocked under these same naltrexone conditions. For oxygen saturation (middle panel, top row, Fig 3), there was a significant three-way interaction between naltrexone, butorphanol and time (F [df=80, 720]=1.43; p=0.05). The blockade of the oxygen saturation effects of butorphanol was linearly related to naltrexone dose. In contrast to the orderly and significant effects on oxygen saturation, outcomes for respiratory rate were a bit more variable. Inspection of the data for respiratory rate (upper right panel, Fig 3) reveal an overall pattern similar to that seen for oxygen saturation -- that is, a reduction produced by butorphanol and some blockade related to naltrexone which was most pronounced at the highest naltrexone doses. However, in this case, there was no statistically significant interaction.
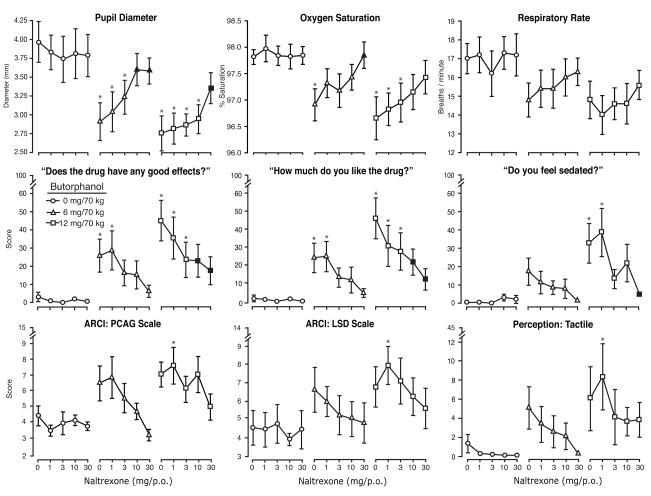
Figure 3.
Data are shown for nine outcomes under all 15 butorphanol/naltrexone dose conditions. The upper panel (from left to right) illustrates three physiological measures (pupil diameter, oxygen saturation and respiratory rate; peak minimum scores). The middle panel illustrates data for three of the visual analog measures (good effects, high and sedated; peak maximum scores). The lower panel illustrates scale scores for the PCAG and LSD scales of the ARCI and the Tactile scale of the Perception Questionnaire (peak maximum scores). Statistical outcomes are shown in Table 1. Each point represents the mean (n=10) ±1 S.E.M. Tukey tests were used for post-hoc analyses. “. Asterisks indicate a significant difference (p<.05) between an active butorphanol/naltrexone dose combination compared to its matched naltrexone dose condition (when given in combination with butorphanol placebo).
Subjective Measures
Visual analog ratings of “good effects” and “liking” for the drug are shown in Figure 3 (left and center panels, middle row). Naltrexone produced a significant and dose-dependent decrease in butorphanol’s acute effects that appears linear upon inspection. For other measures, the profile of drug interaction was somewhat different in that the naltrexone blockade was generally linear for the low dose of butorphanol but less orderly for the high dose of butorphanol. As shown in Figure 3, measures including the visual analog ratings for “sedated,” the PCAG and LSD subscales from the ARCI, and the Tactile subscale from the Perception questionnaire illustrate this pattern of effects. In general, scores declined linearly with naltrexone dose after 6 mg butorphanol for these measures. Following administration of the 12 mg butorphanol dose, there was a small, but reliable (and often statistically significant), increase in scores above placebo levels for each measure when 1 mg of naltrexone was given as the pretreatment. Typically, doses higher than 1 mg of naltrexone attenuated scores on these measures. This profile of action was observed across a number of measures and inspection of the data did not suggest that this was an aberrant finding due to an outlier subject. This same pattern of effects was observed for other measures, including the Visual Scale, Total Perception Scale, General Perception Scale, and the global visual analog ratings of “high” and “any drug effect.” The outcomes for the drug identification questionnaire do not shed any light on this pattern of responding, as the drug category identifications did not substantively change across these dose conditions.
Observer effects
The only measures for which butorphanol produced significant direct effects, (i.e., “nodding” and “spaced/out,”) were reduced by naltrexone, but this reduction reached significance only for the latter measure.
Adverse Event
Volunteer # 12 was a 33-year old African American male who received naltrexone (10 mg) 60 minutes prior to 12 mg/70 kg butorphanol for his seventh experimental session. Approximately 45 min after receiving butorphanol, the volunteer became agitated, removed the physiological monitoring equipment and walked out of the session room. He then appeared to have a tonic-clonic seizure that lasted for 1–2 minutes, which involved bilateral movement of his arms and legs, during which time he was conscious but disoriented. He was admitted to the Emergency Department and had an electroencephalogram, which was normal. He remained in the hospital overnight for observation, after which he was medically cleared by a neurologist and returned to the residential research unit for continued observation. Soon after, he became agitated, refused to be seen for follow-up by the neurologist, and left the study against medical advice. This volunteer had no history of head trauma or seizures and had never taken anticonvulsants. He had previously received the 12mg/70 kg butorphanol dose twice, once in combination with naltrexone placebo and once in combination with naltrexone 1 mg, in sessions prior to this event without incident. He was the last subject enrolled in the study. This event was reported to the FDA, the IRB and the manufacturer of butorphanol.
DISCUSSION
The present findings reveal that butorphanol alone produces a constellation of physiological actions that are mu receptor-mediated (i.e., miosis, respiratory depression) but does not produce those typically associated with kappa receptor-mediated action (diuresis). However, under conditions of differing levels of antagonist blockade, the mu-mediated effects were reduced in an orderly fashion as a function of naltrexone dose, and significant diuresis emerged in response to active butorphanol doses. In contrast to its physiological profile, butorphanol alone produced a composite of subjective responses that included those typically considered mu and kappa-like effects. There was little evidence that the overall contribution of mu- versus kappa-like mood related effects was proportionately changed as a function of naltrexone receptor blockade and, the majority of subjective responses showed a generally linear decline with naltrexone, which raises the possibility that these may be mediated through a single receptor system.
Naltrexone alone produced no detectable pharmacodynamic effects consistent with previous studies which have examined the acute effects of naltrexone in humans (Walsh et al. 1996). In contrast, butorphanol produced robust physiological and subjective changes for both active doses consistent with those previously reported in the literature (Jasinski et al. 1975; Preston et al. 1992; Walsh et al. 2001b; Zacny et al. 1994). Butorphanol produced significant decreases in pupil diameter, respiration rate and oxygen saturation; each of these dose effect functions was flat as both active doses (6 & 12 mg) produced effects of comparable magnitude. These findings are consistent with the characterization of butorphanol as a partial mu agonist. An alternative interpretation is that, at these high doses, there was a limit or ceiling on the magnitude of the responses achievable; however, examination of the respiratory depression data reveal that that maximum reductions were modest (only about 4 breaths/minute), suggesting that this observation is likely attributable to an intrinsic limit on the efficacy of butorphanol.
The data for the subject- and observer-rated responses to butorphanol were concordant with previous studies in humans. Butorphanol produced a constellation of mood and drug effects, including those considered more typical of mu opioid agonists (e.g., “liking for the drug,” “good drug effects,” “nodding”) along with those considered more typical of kappa opioid agonists (increased ratings on the LSD scale, the perceptual disturbance scales, and “coasting/spaced out”). Past studies in humans have suggested that whether butorphanol is deemed more mu- or kappa-like is highly dependent on the comparator control conditions and/or the behavioral arrangement of a given study (see Introduction). The overall pattern of responding (see Fig. 1 & 3) suggested that subjective responses to butorphanol were dose-related; this is more evident for measures such as the visual analog scales where the scores have a wider possible range of variability compared to the scale scores (ARCI and Perception Scale) which have a more limited range and are in contrast to the findings for the physiological outcomes where the two active doses produced effects of comparable magnitude.
The interaction between naltrexone and butorphanol did not reveal a single unifying pattern of responses. Examination of the data shown in Figure 2, reveal that butorphanol alone, over the range of doses tested, produced no significant effect on urine output, although the 6 mg, but not the 12 mg, dose of butorphanol did reduce urine osmolality at the 2 hr collection. Although preclinical studies have reported diuresis after butorphanol dosing in rodents (e.g., (Craft et al. 2000; Horan and Ho 1989), the overall lack of direct effects of butorphanol on urine output in the present study is consistent with previous studies in humans (Walsh et al. 2001b) and non-human primates (Butelman et al. 1995). When naltrexone was given as a pretreatment, butorphanol at both 6 and 12 mg produced significant increases in urine output, which were accompanied by some reduction in urine osmolality; this effect was most prominent during the first two hours after drug administration consistent with other studies of diuresis (e.g., (Peters et al. 1987). For both active doses of butorphanol, the greatest urinary output occurred after pretreatment with the highest naltrexone dose (30 mg). Urinary excretion after treatment with naltrexone at 30 mg in combination with both active doses of butorphanol was significantly greater compared to both 1) when the active butorphanol was given with placebo, and 2) when 30 mg naltrexone was given as a pretreatment to intramuscular placebo. It is worth noting, however, the overall magnitude of the diuresis was less than that observed previously with the full kappa agonist enadoline (Walsh et al. 2001b). The finding that urine volumes were significantly greater when 30 mg naltrexone was given with active butorphanol in comparison to any of the control conditions suggests that naltrexone blockade led to butorphanol-induced diuresis, and that this is likely a kappa receptor-mediated effect rather than simple reversal of mu-receptor mediated urinary retention. This finding is consistent with those from rhesus monkeys demonstrating that butorphanol can produce diuresis but only in the presence of mu-receptor blockade (in that case, only by the irreversible antagonist, clocinnamox) (Vivian et al. 1999).
Other physiological responses to butorphanol (i.e., miosis and reductions in indices of respiratory function) showed linear reversal with increasing naltrexone doses suggesting that these mu-mediated actions were competitively blocked. For the most part, reversal was nearly complete at the highest naltrexone dose (30 mg), although there was modest evidence for the 12 mg dose of butorphanol to exert some activity under this pretreatment condition (i.e., responses were not comparable to baseline). Because kappa opioid agonists, as part of their diuretic activity, can produce sweating, and because we observed profuse sweating in an earlier study with enadoline (Walsh et al. 2001b), we hoped to quantify increases in sweating by employing a measurement of electrodermal activity -- galvanic skin resistance (Gutrecht 1994). We hypothesized that an increase in surface water could lead to decreased electrical resistance (i.e., improved conductance). However, on this experimental measure, no systematic effects of butorphanol were observed when given either alone or in combination with naltrexone.
Naltrexone produced an orderly and dose-dependent reduction of those subjective responses to butorphanol typical of mu opioid action. For example, ratings of “drug liking” (Fig. 3) illustrate this pattern of blockade. Interestingly, blockade of the lower dose (6 mg) was nearly complete with naltrexone 30 mg, while the effects of the 12 mg butorphanol dose were not fully suppressed by naltrexone 30 mg. For subjective measures more typically considered reflective of kappa activity (perceptual disturbances and increased scores on the LSD scale), the naltrexone blockade was also generally dose-dependent. While there was a reliable and, sometimes significant, increase in ratings when 1 mg of naltrexone was administered prior to the 12 mg dose of butorphanol, the increase was modest and did not occur under any other naltrexone blockade condition. These data suggest that the constellation of subjective effects of butorphanol may, in fact, be mediated by mu receptors with little kappa involvement. Alternatively, it is possible that the kappa-like effects of butorphanol are relatively modest over this range of supratherapeutic doses and that the doses of naltrexone were not appropriate for producing selective mu blockade. Inclusion of a selective kappa agonist may have helped elucidate this finding; however, none were available for human testing at the time that this study was conducted. One past study did demonstrate selective blockade with naltrexone in combination with the mixed opioid pentazocine (Preston and Bigelow 1993). In that study, a single naltrexone dose (12.5 mg) led to increased scores on measures of kappa-like effects (e.g., the LSD scale of the ARCI), which were not evident when the same dose of pentazocine 60 mg) was co-administered with either placebo or a high naltrexone dose (25 mg).
In summary, butorphanol was well tolerated in this population of sporadic opioid abusers even at the supratherapeutic doses tested here. When given alone, the mu receptor mediated physiological effects of butorphanol were predominant, while the kappa-like effects were virtually absent. However, in combination with naltrexone blockade, butorphanol produced significant diuresis suggesting that butorphanol does possess physiologically relevant kappa agonist activity at these doses, which may be suppressed in the presence of mu agonist action (in this case, possibly opposing actions of urinary retention versus diuresis). In contrast, butorphanol produced a constellation of mood and drug effects historically considered to be mediated by mu and kappa receptors; however, the dysphoric effects of butorphanol were not as profound as those produced by selective kappa agonists. Moreover, naltrexone produced a dose-dependent blockade of these responses suggesting that these effects traditionally attributed to two receptor systems were not readily dissociated from one another leading the possibility that they are mediated through a single receptor system. Finally, the urinalysis data do suggest that naltrexone antagonism can be used to unveil the intrinsic kappaergic activity of butorphanol in humans.
Acknowledgments
This project was supported by funding from the National Institute on Drug Abuse (R01DA100753; R01DA016718 SLW). This study complies with the current laws of the United States of America.
The authors thank the staff at the Behavioral Pharmacology Research Unit at Johns Hopkins University and the staff at the Human Behavioral Science Center at the University of Kentucky for their assistance with the conduct of this study, data analysis and manuscript preparation. This project was supported by funding from the National Institute on Drug Abuse (R01DA100753 SLW; R01DA016718 SLW; K02 DA00332 ECS). The authors did not have any financial conflicts of interest related to this study. This study complies with the current laws of the United States of America.
References
- Abramson HA, Jarkvik ME, Kaufman MR, Kornetsky C, Levine A, Wagner M. Lysergic acid diethylamide (LSD-25) I. Physiological and perceptual responses. Journal of Psychology. 1955;30:3–60. [Google Scholar]
- Ananthan S, Kezar HS, Carter RL, Saini SK, Rice KC, Wells JL, Davis P, Xu H, Dersch CM, Bilsky EJ, Porreca F, Rothman RB. Synthesis, opioid receptor binding, and biological activities of naltrexone-derived pyrido- and pyridomorphinans. J Med Chem. 1999;42:3527–3538. doi: 10.1021/jm990039i. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Winger G, Zernig G, Woods JH. Butorphanol: Characterization of agonist and antagonist effects in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1995;272:845–853. [PubMed] [Google Scholar]
- Commiskey S, Fan L-W, Ho IK, Rockhold RW. Butorphanol: Effects of a prototypical agonist-antagonist analgesic on k-opioid receptors. Journal of Pharmacological Sciences. 2005;98:109–116. doi: 10.1254/jphs.crj05001x. [DOI] [PubMed] [Google Scholar]
- Craft RM, McNiel DM. Agonist/antagonist properties of nalbuphine, butorphanol and (−)-pentazocine in male vs. female rats. Pharmacology, Biochemistry and Behavior. 2003;75:235–245. doi: 10.1016/s0091-3057(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Craft RM, Ulibarri CM, Raub DJ. Kappa opioid-induced diuresis in female vs. male rats. Pharmacology, Biochemistry and Behavior. 2000;65:53–59. doi: 10.1016/s0091-3057(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Dershwitz M, Rosow CE, DiBiase CE, Zaslavsky A. Comparison of the sedative effects of butorphanol and midazolam. Anesthesiology. 1991;74:717–724. doi: 10.1097/00000542-199104000-00016. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradshky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. Journal of Pharmacology and Experimental Therapeutics. 1996;278:1121–1127. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IV Axis I disorders. New York State Psychiatric Institute; 1996. [Google Scholar]
- Fraser HF, Van Horn GG, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) “Attitude of opiate addicts toward opiate-like drugs, (B) A short-term “direct” addiction test. Journal of Pharmacology and Experimental Therapeutics. 1961;133:371–378. [PubMed] [Google Scholar]
- Gharagozlou P, Demirci H, Clark JD, Lameh J. Activation profiles of opioid ligands in HEK cells expressing delta opioid receptors. Biomed Central Neuroscience. 2002:18. doi: 10.1186/1471-2202-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JC, Benfield P, Goa KL. Transnasal butorphanol. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute pain management. Drugs. 1995;50:157–175. doi: 10.2165/00003495-199550010-00010. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Stitzer ML. Butorphanol agonist effects and acute physical dependence in opioid abusers: Comparison with morphine. Drug and Alcohol Dependence. 1998;53:17–30. doi: 10.1016/s0376-8716(98)00104-5. [DOI] [PubMed] [Google Scholar]
- Gutrecht JA. Sympathetic skin response. Journal of Clinical Neurophysiology. 1994;1:519–524. doi: 10.1097/00004691-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Heel RC, Brogden RN, Speight TM, Avery GS. Butorphanol: A review of its pharmacological properties and therapeutic efficacy. Drugs. 1978;16:473–505. doi: 10.2165/00003495-197816060-00001. [DOI] [PubMed] [Google Scholar]
- Horan PJ, Ho IK. Comparative pharmacological and biochemical studies between butorphanol and morphine. Pharmacology, Biochemistry and Behavior. 1989;34:847–854. doi: 10.1016/0091-3057(89)90284-0. [DOI] [PubMed] [Google Scholar]
- Houde RW, Wallenstein SL, Rogers A, Kaiko RF. Annual report of the analgesic studies section of the Memorial Sloan-Kettering Cancer Center. 1976:149–168. [Google Scholar]
- Hunter JC, Leighton GE, Meecham KG, Boyle SJ, Horwell DC, Rees DC, Hughes J. CI-977, a novel and selective agonist for the kappa-opioid receptor. Br J Pharmacol. 1990;101:183–9. doi: 10.1111/j.1476-5381.1990.tb12110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell H, Bellevile RE, Fraser HF, Wikler A, Logan CR. Studies on lysergic acid diethylamide (LSD-25). I. Effects in former morphine addicts and development of tolerance during chronic intoxication. Archives of General Psychiatry. 1956;76:468–478. [PubMed] [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick J, Gorodetzky C, Cone E, Kay D. Progress report from the clinical pharmacology section of the NIDA Addiction Research Center. 37th Annual Meeting, The Committee on Problems of Drug Dependence; Washington, D.C: National Research Council, National Academy of Sciences; 1977. pp. 133–168. [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick JS, Clark SC. Progress report on studies from the Clinical Pharmacology Section of the Addiction Research Center. Proceedings of the Thirty-Seventh Annual Scientific Meeting Committee on Problems of Drug Dependence; Washington, D.C. 1975. pp. 136–161. [Google Scholar]
- Ko M-C, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Kumor KM, Haertzen CA, Johnson RE, Kocher TR, Jasinski DR. Human psychopharmacology of ketocyclazocine as compared with cyclazocine, morphine and placebo. Journal of Pharmacology and Experimental Therapeutics. 1986;238:960–968. [PubMed] [Google Scholar]
- Leander JD. Evidence that nalorphine, butorphanol and oxilorphan are partial agonists at the k-opioid receptor. Eur J Pharmacol. 1983a;86:467–470. doi: 10.1016/0014-2999(83)90198-x. [DOI] [PubMed] [Google Scholar]
- Leander JD. Evidence that nalorphine, butorphanol, and oxilorphan are partial agonists at the k-opioid receptor. European Journal of Pharmacology. 1983b;86:467–470. doi: 10.1016/0014-2999(83)90198-x. [DOI] [PubMed] [Google Scholar]
- Leander JD, Zerbe RL, Hart JC. Diuresis and suppression of vasopressin by kappa opioids: comparison with mu and delta opioids and clonidine. Journal of Pharmacology and Experimental Therapeutics. 1985;234:463–469. [PubMed] [Google Scholar]
- Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29:1207–1211. [PubMed] [Google Scholar]
- Loder E. Post-marketing experience with an opioid nasal spray for migraine: lessons for the future. Cephalalgia. 2006;26:89–97. doi: 10.1111/j.1468-2982.2005.00951.x. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan BS, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clinical Pharmacology and Therapeutics. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Pachter IJ, Evens RP. Butorphanol. Drug and Alcohol Dependence. 1985;14:315–338. doi: 10.1016/0376-8716(85)90065-1. [DOI] [PubMed] [Google Scholar]
- Peters GR, Ward NJ, Antal EG, Lai PY, deMaar EW. Diuretic actions in man of a selective kappa opioid agonist: U-62,066E. Journal of Pharmacology and Experimental Therapeutics. 1987;240:128–131. [PubMed] [Google Scholar]
- Pircio AW, Gylys JA, Cavanagh RL, Buyniski JP, Bierwagen ME. The pharmacology of butorphanol, a 3, 14-dihydroxymorphinan narcotic antagonist analgesic. Archives of International Pharmacodynamics and Therapy. 1976;220:231–257. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Differential naltrexone antagonism of hydromorphone and pentazocine effects in human volunteers. Journal of Pharmacology and Experimental Therapeutics. 1993;264:813–823. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Effects of agonist-antagonist opioids in humans trained in a hydromorphone/not hydromorphone discrimination. Journal of Pharmacology and Experimental Therapeutics. 2000;295:114–124. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA. Drug discrimination in human postaddicts: Agonist-antagonist opioids. Journal of Pharmacology and Experimental Therapeutics. 1989;250:184–196. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Discrimination of butorphanol and nalbuphine in opioid-dependent humans. Pharmacology, Biochemistry and Behavior. 1990;37:511–522. doi: 10.1016/0091-3057(90)90021-9. [DOI] [PubMed] [Google Scholar]
- Preston KL, Liebson IA, Bigelow GE. Discrimination of agonist-antagonist opioids in humans trained on a two-choice saline-hydromorphone discrimination. Journal of Pharmacology and Experimental Therapeutics. 1992:261. [PubMed] [Google Scholar]
- Rimoy GH, Bhaskar NK, Wright DM, Rubin PC. Mechanism of diuretic action of spiradoline (U-62066E)--a kappa opioid receptor agonist in the human. Br J Clin Pharmacol. 1991;32:611–5. doi: 10.1111/j.1365-2125.1991.tb03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slizgi GR, Ludens JH. Studies on the nature and mechanism of the diuretic activity of the opioid analgesic ethylketocyclazocine. J Pharmacol Exp Ther. 1982;220:585–91. [PubMed] [Google Scholar]
- Vivian JA, Deyoung MB, Sumpter TL, Traynor JR, Lewis JW, Woods JH. k-Opioid receptor effects of butorphanol in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1999;290:259–265. [PubMed] [Google Scholar]
- Vonvoigtlander PF, Lahti RA, Ludens JH. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Evaluation of enadoline and butorphanol effects on cocaine self-administration and cocaine pharmacodynamics in humans. Drug and Alcohol Dependence. 2001a;60:S231. [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology. 2001b;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. Journal of Pharmacology and Experimental Therapeutics. 1996;279:524–538. [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Thapar P, Coalson DW, Flemming D, Thompson WK. Comparing the subjective, psychomotor and physiological effects of intravenous butorphanol and morphine in healthy volunteers. Journal of Pharmacology and Experimental Therapeutics. 1994;270:579–588. [PubMed] [Google Scholar]
- Zhu J, Luo L-Y, Li J-G, Chen C, Liu-Chen L-Y. Activation of the cloned human kappa opioid receptor by agonists enhances [35]GTPgammaS binding to membranes: Determination of potencies and efficacies of ligands. Journal of Pharmacology and Experimental Therapeutics. 1997;282:676–684. [PubMed] [Google Scholar]