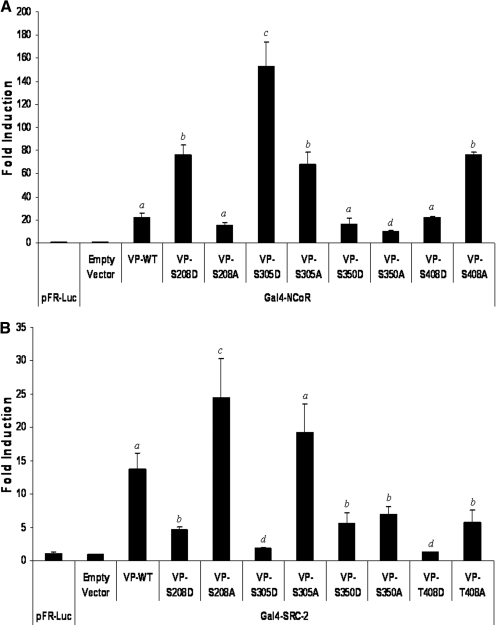
Fig. 5.
Analysis of mutant PXR proteins ability to interact with protein cofactors. CV-1 cells were transfected with the pFR-Luc reporter gene construct and expression vectors encoding GAL4-cofactor fusion proteins and VP16-wild-type or -mutant PXR fusion proteins. Luciferase activity was observed 48 h after transfection. A, phosphomimetic mutations at Ser208 and Ser305 increase the strength of interaction between PXR and the corepressor NCoR. Phospho-deficient mutations at positions Ser305 and Ser408 also increase the strength of interaction between PXR and the corepressor NCoR. B, phosphomimetic mutations at positions Ser208, Ser305, Ser350, and Thr408 decrease the strength of interaction between PXR and the coactivator protein SRC2. Phosphodeficient mutation at position Ser208 increases interaction with SRC2, whereas the S350A and T408A mutations decrease strength of association with SRC2. The data are normalized to β-galactosidase activity and represented as fold induction above reporter gene alone ± S.D. (n = 4). Letters different from each other represent statistically different groups (p < 0.05).