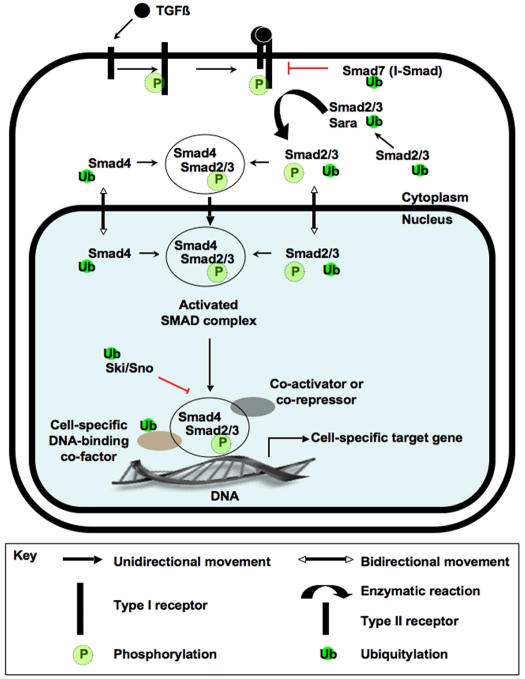
Fig. 1.
TGFβ signal transduction. Events downstream of ligand-receptor binding in a TGFβ-responsive cell. When a TGFβ ligand binds to a type II transmembrane receptor serine-threonine kinase, the receptor recruits a type I receptor and phosphorylates it (P) within a serine/threonine-rich region. Serine-threonine phosphorylation stimulates the type I receptor to phosphorylate one or more C-terminal serines in a receptor-associated Smad (R-Smad), such as Smad2 and Smad3 (Smad2/3). At the membrane, Smad anchor for receptor activation (Sara) acts as an adapter and facilitates the interaction between R-Smads and their type I receptor. The phosphorylation of Smad2/3 stimulates their translocation into the nucleus, where they form a heteromeric complex with the common-mediator Smad (Co-Smad), Smad4. This multi-Smad complex then regulates the expression of TGFβ target genes in cooperation with tissue-specific activators and repressors. Solid arrows represent the movement of information and protein subcellular localization (see key). Red T-bars represent the activity of proteins that block information transfer, such as I-Smads (Smad7) and members of the Ski/Sno family. The regulation of pathway components by polyubiquitylation (Ub) is also shown.