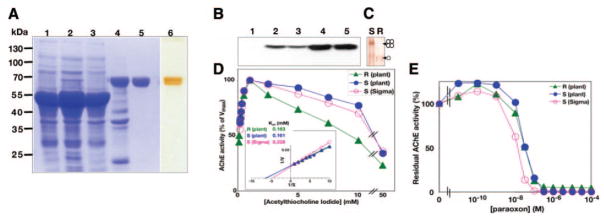
Figure 2.
Characterization of plant-produced AChE-RER. A) Fractions from successive purification steps were subjected to SDS-PAGE and visualized by Coomassie staining (lanes 1–5) or silver staining (lane 6) as follows: crude WT extract (lane 1), crude 2D extract (lane 2), procainamide affinity chromatography flow-through (lane 3), eluate after extensive dialysis (lane 4), final product after anion-exchange chromatography and concentration (lanes 5, 6). B) The above fractions were subjected to immunoblot analysis (lanes 1–5) with AChE-specific Abs. C) Nondenaturing PAGE, followed by staining for ChE activity analysis of plant-derived AChE-S (S) and AChE-RER. D) Plant-produced AChE-RER (R) displays equivalent Km and substrate inhibition compared with mammalian cell culture (S, Sigma) or plant produced AChE-S (S, plant). Insert: Lineweaver-Burke analysis (insert). E) Residual activities of plant-derived AChE-RER and AChE-S and mammalian cell culture-derived AChE-S were assayed in the presence of the indicated concentration of the OP paraoxon.