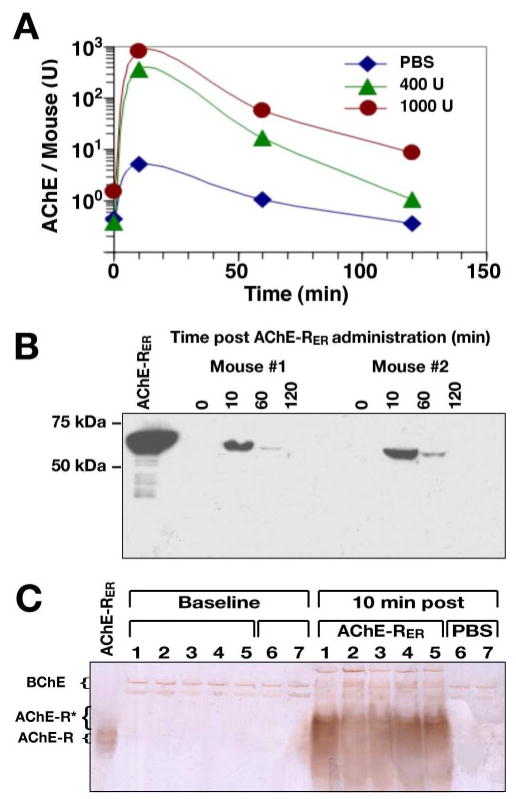
Figure 3.
Pharmacokinetics of plant-produced AChE-RER. A) AChE-RER clearance profile. Groups of 5 mice were injected with either 400 U, 1000 U of plant-produced AChE-RER or an equivalent volume of saline. Plasma samples were collected and assayed for AChE activity in the presence of Iso-OMPA, a selective BChE inhibitor. B) Immunoblot detection of intact circulating AChE-RER. Plasma protein samples from 2 mice injected with 400 U AChE-RER were resolved by SDS-PAGE and subjected to immunoblotting. A 1.3 μg sample of pure plant-derived AChE-RER was resolved alongside for comparison. C) Circulating AChE-RER displays decreased migration in nondenaturing PAGE. Plasma samples from 5 mice injected with 400 U AChE-RER and 2 mice injected with PBS were prepared and subjected to nondenaturing PAGE, followed by staining of catalytically active AChE. Fast migrating AChE-R monomers appeared only after injection of the recombinant enzyme. In panels B, C, purified plant-produced AChE-RER was resolved alongside the serum samples for comparison.