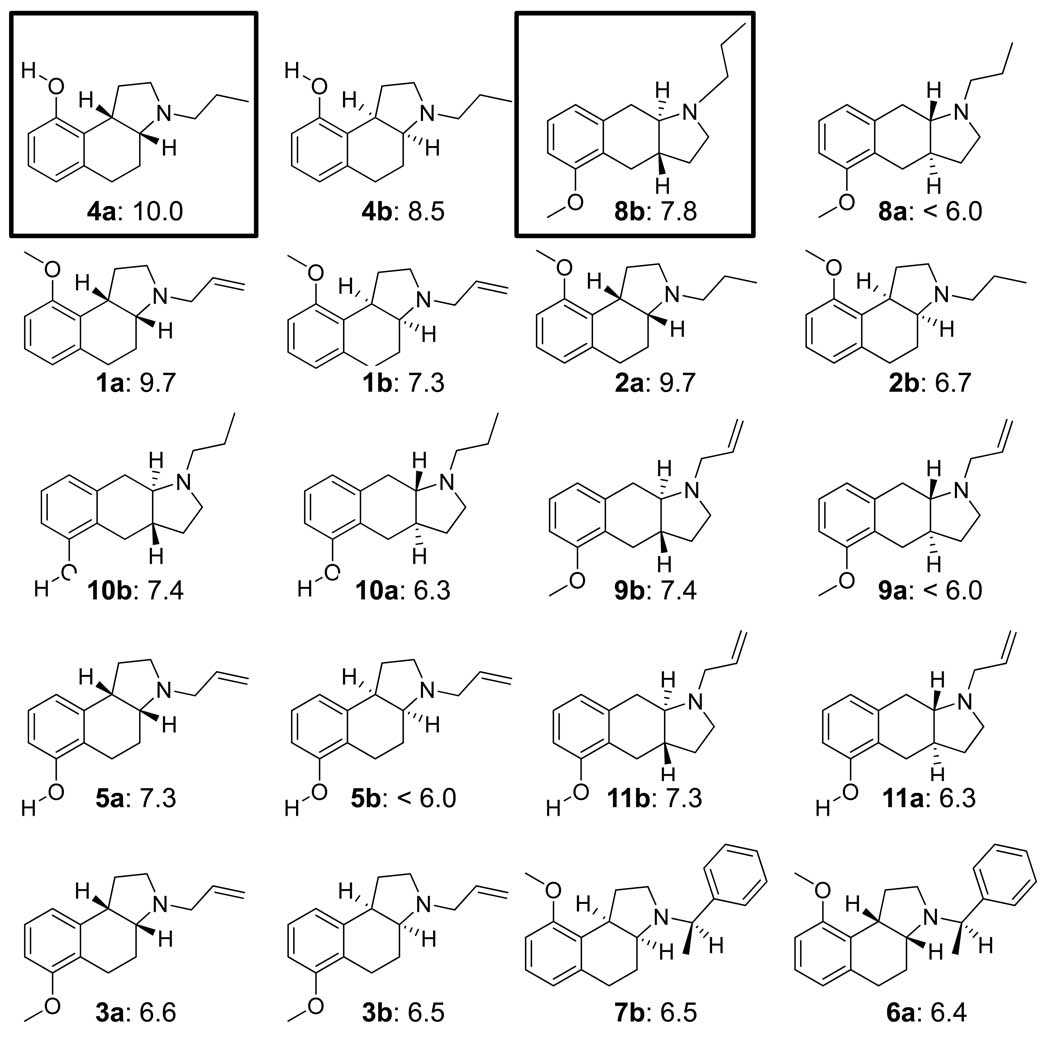
Figure 1.
Structures and experimentally determined pKd of training ligands for 5HT1a. The molecules are shown as enantiomeric pairs, with a/b designating the isomers. The boxed ligands in the top row were the most active of the two scaffold types and were used to guide initial alignments for pocket induction (see Figure 3). The remaining molecules are shown by decreasing potency of the more active isomer. The differences in potency among isomers range from nearly 2 log units to zero. There is a complex interaction between sidechains, scaffold, and chirality that is difficult to model without contemplation of a full 3D model. Compounds numbers are as in ref 16.