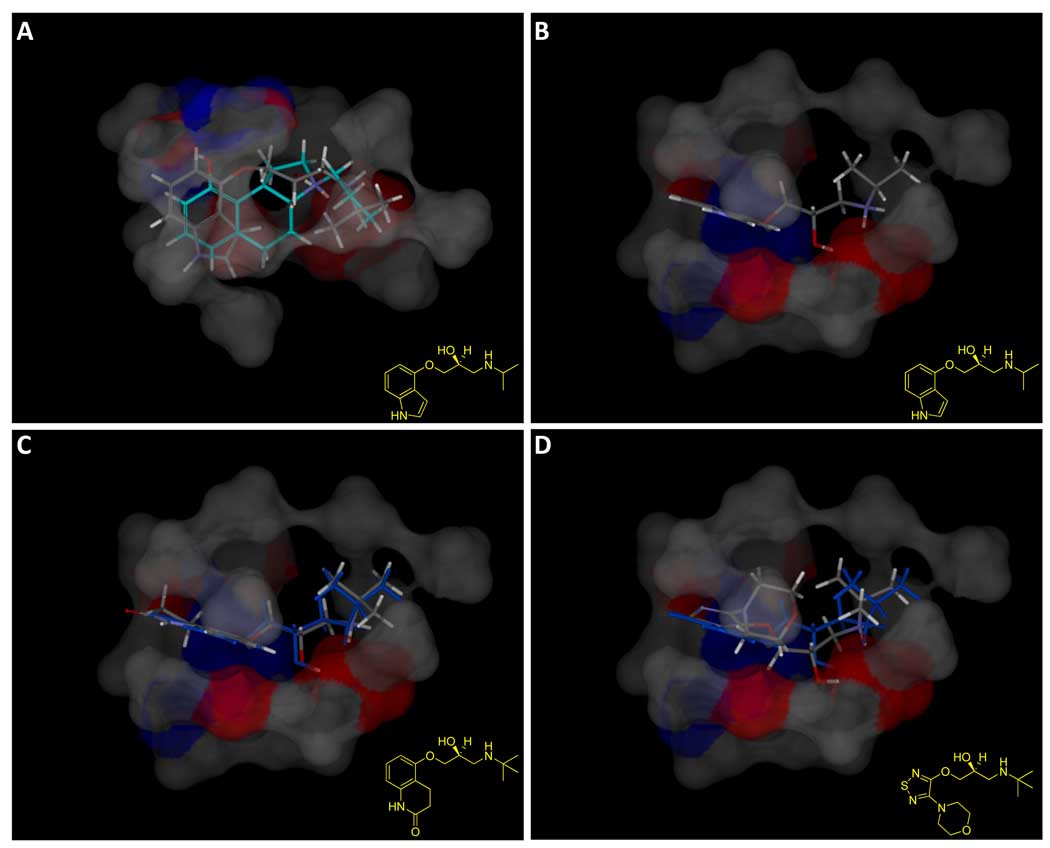
Figure 12.
The beta adrenergic antagonist pindolol was among the accurate predictions from the Organon binding data, shown in Panel A along with 4a (cyan carbons) and the pocketmol surface colored to reflect polarity. Panel B shows the view from the bottom, without 4a. The interaction between the amine and hydroxyl to the large negative pocket surface is apparent, as is the interaction of the ether oxygen with the donor surface. Panel C shows carteolol (atom color), which mimics the binding mode of pindolol (shown in blue), and also scores greater than 7.0. The ether oxygens of pindolol and carteolol are able to act as the critical acceptor functionality within the 5HT1a pocket while making proper interactions with their protonated amines. Timolol (Panel D, atom color with pindolol in blue), by contrast, while being a structural analog, is correctly predicted to be inactive (predicted pKd of 5.2). It appears that the additional bulk and non-planarity of the morpholine renders timolol unable to make the correct interactions. See text for additional details on adrenergic ligand predictions.