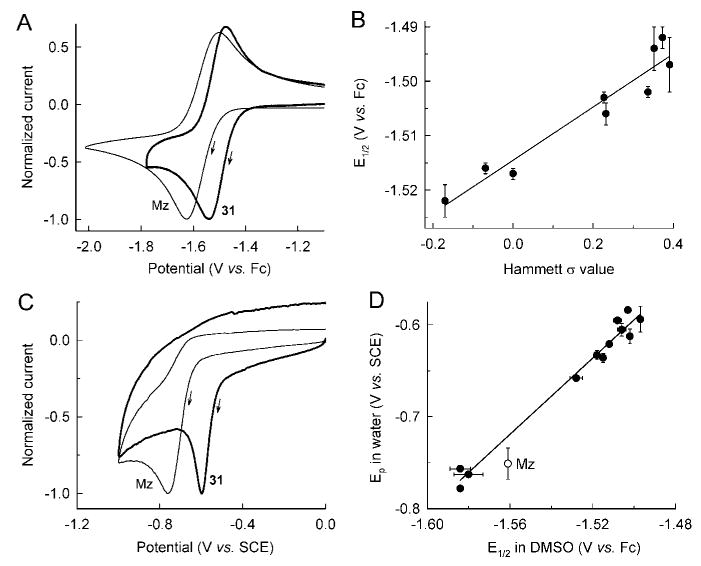
Figure 4.
Electrochemistry of new 5-NI compounds. 5-NIs were subjected to cyclic voltammetry in DMSO (A) or pH 7.0 water (C). Representative scans for Mz and the 3-thiophene compound 31 are shown. Potential is given relative to ferrocene (Fc) in DMSO or a saturated calomel electrode (SCE) in water. Current was normalized against peak cathodic current for each scan. The arrows indicate the scan direction. A summary of the electrochemical data is given in Table 6. E1/2 values in DMSO were plotted against Hammett σ values of the 2-alkenylphenyl 5-NI compounds 5, 7, 8, 11, 12, 14, 15, 18, and 19 (B). E1/2 values in DMSO were also plotted against Ep values in water for selected 5-NIs using the data in Table 6 (D). Data in (B) and (D) are the mean ± SE of three to four experiments.