Table 6.
Electrochemical Properties of Modified 5-NIs
 | |||||
|---|---|---|---|---|---|
| mean ± SE |
|||||
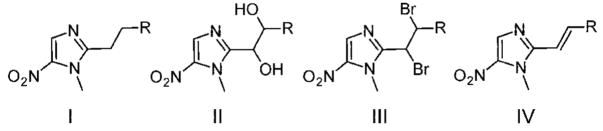
| groupa | name | structure | R | E1/2 in DMSO (V vs Fc)b | Ep in water (V vs SCE)b,c |
| 40 | I | phenyl | −1.593 ± 0.002 | ND | |
| 41 | I | 4-methylphenyl | −1.592 ± 0.003 | ND | |
| 42 | I | 4-bromophenyl | −1.592 ± 0.003 | ND | |
| 22 | IV | 2,3,4-trimethoxyphenyl | −1.584 ± 0.005 | −0.757 ± 0.003 | |
| 27 | IV | 1,3-benzodioxolyl | −1.584 ± 0.001 | −0.778 ± 0.002 | |
| 20 | IV | 3-methoxyphenyl | −1.580 ± 0.007 | −0.763 ± 0.001 | |
| Mz | −1.561 ± 0.001 | −0.751 ± 0.017 | |||
| 1-methyl-5-NI | −1.557 ± 0.001 | ND | |||
| 34 | II | 4-methylphenyl | −1.557 ± 0.001 | ND | |
| 35 | II | 4-bromophenyl | −1.544 ± 0.001 | ND | |
| A | 9 | IV | 2,4,6-trimethylphenyl | −1.529 ± 0.001 | ND |
| 30 | IV | 2-thiopheneyl | −1.528 ± 0.003 | −0.658 ± 0.001 | |
| 8 | IV | 4-methylphenyl | −1.522 ± 0.003 | ND | |
| C | 23 | IV | 4-diethoxymethylphenyl | −1.518 ± 0.003 | ND |
| 5 | IV | phenyl | −1.517 ± 0.001 | −0.634 ± 0.004 | |
| 7 | IV | 3-methylphenyl | −1.516 ± 0.001 | −0.635 ± 0.003 | |
| 6 | IV | 2-methylphenyl | −1.512 ± 0.001 | −0.621 ± 0.001 | |
| C | 24 | IV | 2-biphenyl | −1.512 ± 0.001 | ND |
| 26 | IV | 2-napthyl | −1.509 ± 0.001 | ND | |
| 31 | IV | 3-thiopheneyl | −1.508 ± 0.002 | −0.595 ± 0.001 | |
| C | 12 | IV | 4-bromophenyl | −1.506 ± 0.002 | −0.605 ± 0.007 |
| 29 | IV | 2-furanyl | −1.504 ± 0.003 | ND | |
| A | 15 | IV | 4-chlorophenyl | −1.503 ± 0.001 | −0.584 ± 0.001 |
| 25 | IV | 1-napthyl | −1.502 ± 0.004 | ND | |
| A | 19 | IV | 3-fluorophenyl | −1.502 ± 0.001 | −0.613 ± 0.008 |
| C | 28 | IV | 1-methylimidazolyl | −1.497 ± 0.001 | −0.594 ± 0.014 |
| A | 11 | IV | 3-bromophenyl | −1.497 ± 0.005 | ND |
| 18 | IV | 3-iodophenyl | −1.494 ± 0.004 | ND | |
| B | 33 | III | 4-bromophenyl | −1.493d ± 0.004 | ND |
| 14 | IV | 3-chlorophenyl | −1.492 ± 0.002 | ND | |
| 3 | III | 4-methylphenyl | −1.489d ± 0.001 | ND | |
| 21 | IV | 4-trifluoromethoxyphenyl | −1.487 ± 0.001 | ND | |
| 13 | IV | 2-chlorophenyl | −1.486 ± 0.004 | ND | |
| B | 10 | IV | 2-bromophenyl | −1.481 ± 0.001 | ND |
| 16 | IV | 2,4-dichlorophenyl | −1.469 ± 0.002 | ND | |
Redox activation potential was determined by cyclic voltammetry in DMSO or water (pH 7.0). Half-wave potential (E1/2) of the one-electron transfer reaction (0/−1 reduction) was determined in DMSO, with ferrocene (Fc) as the internal reference. Peak potential, Ep, for the irreversible first electron reduction step was determined in water, using a saturated calomel electrode (SCE) as standard. Data are mean ± SE of three to four separate experiments.
ND, not determined.
The E1/2 values for these compounds are for the reversible wave, but given the different electrochemical response, these values can not be directly compared to the other E1/2 values.
