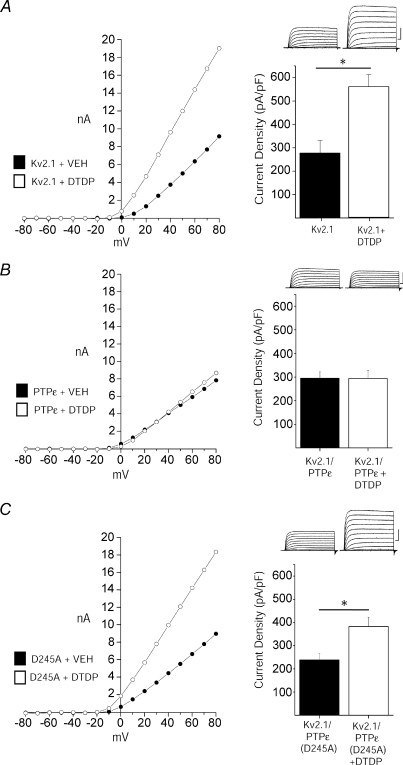
Figure 4. Cyt-PTPɛ blocks apoptotic K+ current enhancement.
A, DTDP induces current enhancement in Kv2.1-expressing CHO cells. Left, representative steady-state current–voltage relationship of Kv2.1-expressing CHO cells recorded following vehicle (filled) and DTDP treatment (open) conditions (30 μm for 5 min). Right, representative whole-cell currents and mean ±s.e.m. current densities from Kv2.1-expressing CHO cells recorded following vehicle (n= 15) and DTDP (n= 12) treatment conditions; bar graphs are the same as those shown in Fig. 1A and B for wild-type Kv2.1. B, Cyt-PTPɛ and Kv2.1 co-expression blocks apoptotic K+ current densities in CHO cells. Left, representative steady-state current–voltage relationship of Kv2.1- and Cyt-PTPɛ-expressing CHO cells recorded following vehicle (filled) and DTDP treatment (open) conditions. Currents were obtained 3 h post-oxidative injury. Right, representative whole-cell currents and mean ±s.e.m. current densities from Kv2.1- and Cyt-PTPɛ-expressing CHO cells recorded under control (n= 16, filled) and DTDP treatment (n= 16, open) conditions. C, enzymatic activity of Cyt-PTPɛ is required for inhibition of apoptotic K+ current enhancement. Left, representative steady-state current–voltage relationship of Kv2.1- and Cyt-PTPɛ(D245A)-expressing CHO cells recorded under control (filled) and DTDP treatment (open) conditions. Right, representative whole-cell currents and mean ±s.e.m. current densities from Kv2.1- and Cyt-PTPɛ(D245A)-expressing CHO cells recorded under control (n= 16, filled) and DTDP treatment (n= 16, open) conditions (*P < 0.01; 2-tailed t test). Current density was calculated as the steady-state K+ current evoked by a single voltage step to +10 mV from a holding potential of −80 mV normalized to cell capacitance.