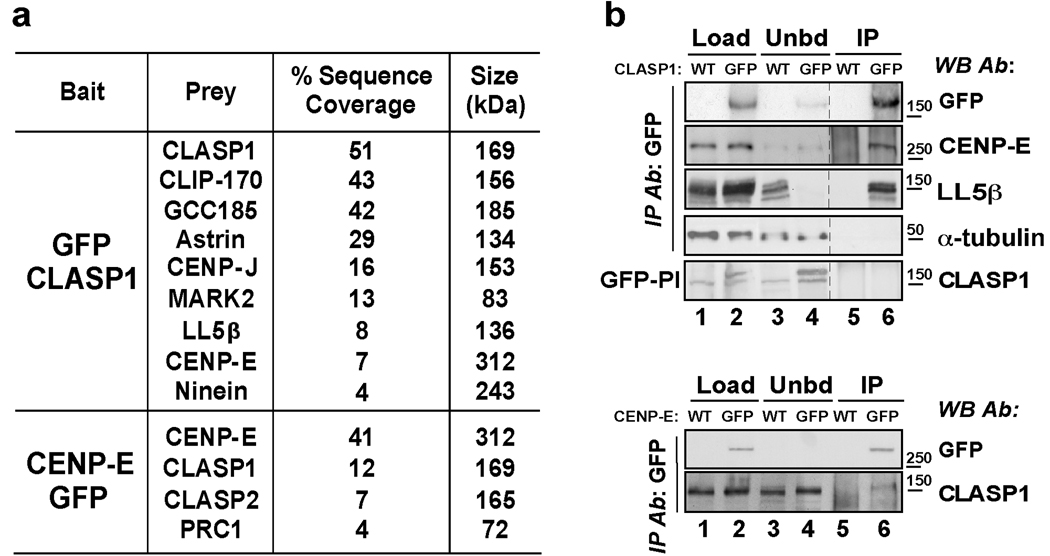
Figure 1. Human CLASP1 interacts with CENP-E.
(a) Mass spectrometry analysis of affinity purified GFP-(LAP)-CLASP1 and CENP-E-GFP identifies novel protein interactions. Polypeptides identified (Prey) and the percentages of the relative sequence coverage are indicated. A complete list of the polypeptides identified during this analysis is given in Table S1. (b) Anti-GFP Immunoprecipitation from mitotic enriched HeLa cells stably expressing GFP-(LAP)-CLASP1 or CENP-E-GFP. Native protein extracts (Load) obtained from the indicated cell lines, unbound proteins (Unbd) and immunoprecipitations (IP) were subjected to western blot analysis with the indicated antibody. Immunoprecipitations were blotted for LL5β and α-tubulin as positive and negative controls. Immunoprecipitations performed using anti-GFP pre-immunization serum (GFP-PI) as precipitating antibody were analyzed by western blotting with rabbit anti-CLASP1 antibody. Quantification of CENP-E levels in the GFP-CLASP1 immunoprecipitation revealed +131% increase relative to control. Quantification of CLASP1 levels in the CENP-E-GFP immunoprecipitation revealed +135% increase relative to control.