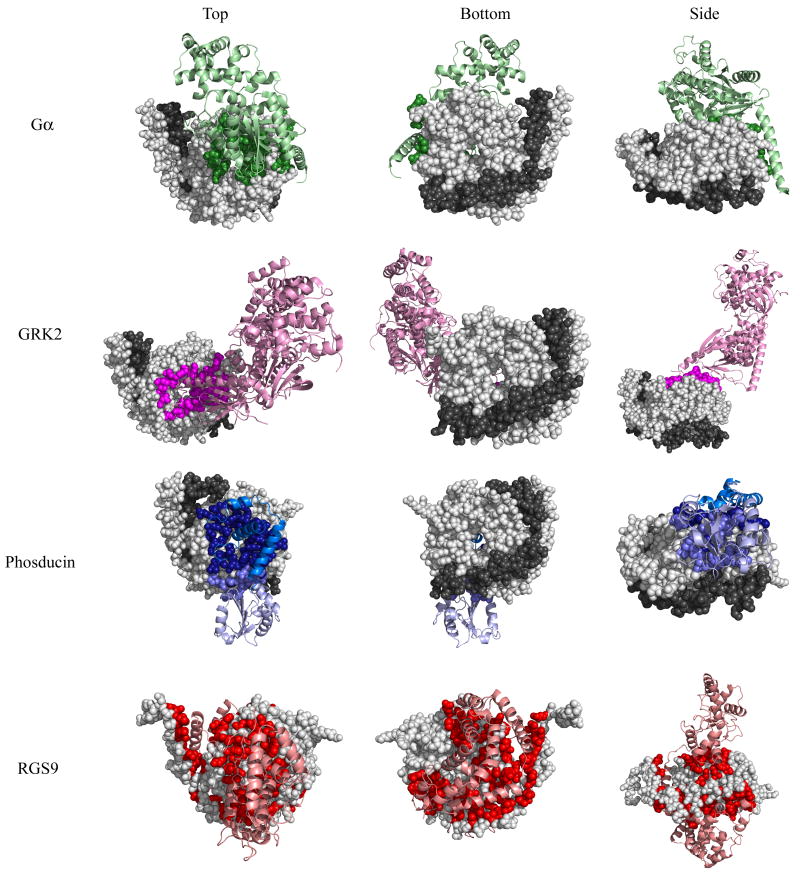
Fig 1. Binding interfaces of four Gβ-interacting proteins as determined by crystal structures.
Top, bottom, and side views of Gβ or the Gβγ dimer (Gβ in light gray spheres, Gγ in dark gray spheres) bound to four different interacting proteins (ribbon): Gβ1-Gα in green (1got.pdb), Gβ1-GRK2 in magenta (1omw.pdb), Gβ1-phosducin in blue (N-terminal domain is dark, C-terminal domain is light, 1a0r.pdb), and Gβ5-RGS9 in red (2pbi.pdb). Gβ residues that contact each interacting protein are colored accordingly. Binding contacts were determined by evaluating the solvent accessibility difference between single molecules and those in complex.