Abstract
Objective
We conducted a population-based pharmacokinetic study to assess blood levels and elimination of mercury following vaccination of premature infants born at ≥ 32 and < 37 weeks of gestation and with birth weight ≥ 2000 but < 3000 grams.
Study design
Blood, stool, and urine samples were obtained pre-vaccination and 12 hours to 30 days post-vaccination from 72 premature newborns. Total mercury levels were measured by atomic absorption.
Results
The mean ± standard deviation (SD) birth weight was 2.4 ±0.3 Kg for the study population. Maximal mean ± SD blood mercury levels was 3.6 ± 2.1 ng/mL occurring at 1 day after vaccination, maximal mean ± SD stool mercury levels were 35.4 ± 38.0 ng/g, occurring on day 5 after vaccination, and urine mercury levels were mostly non-detectable. The blood mercury half-life was calculated to be 6.3 (95% CI:3.85-8.77) days and mercury levels returned to pre-vaccination levels by day 30.
Conclusions
The blood half-life of intramuscular ethyl mercury from thimerosal in vaccines given to premature infants is substantially shorter than that of oral methyl mercury in adults. Because of the differing pharmacokinetics, exposure guidelines based on oral methyl mercury in adults may not be accurate for children who receive thimerosal-containing vaccines.
The recommendation of the American Academy of Pediatrics (AAP)1 the Center for Disease Control (CDC) and the Food and Drug Administration (FDA)2 to reduce or remove thimerosal as a preservative in vaccine administered to children was made in 1999 and implemented by 2001. The recommendations were made because of expanding infant immunization schedule in the US, which would permit the injection of up to 62.5 μg of ethyl mercury (the active ingredient of thimerosal) at a single health care visit and up to 187.5 μg cumulatively across all health care visits during the first 6 months of life. Lacking specific data on ethyl mercury metabolism and half-life in infants receiving vaccines containing ethyl mercury, the AAP, CDC and FDA relied on metabolism and half-life data of another form of mercury, i.e., methyl mercury, as is present in fish and is ingested orally by adults. Calculations of possible persistence of ethyl mercury in the body of a low birth weight or prematurely born infant based on the ethyl mercury content of all the infant vaccines recommended in the first six months of life led to the conclusion that levels of ethyl mercury from injections could exceed those recommended by the Environmental Protection Agency for oral consumption of methyl mercury in fish.3
In two prior studies, we demonstrated that blood mercury levels were low after vaccines containing thimerosal were given to full term newborn, 2-month, and 6-month old infants, and that mercury was detected in increased concentration in the stools following vaccination, suggesting elimination by the gastrointestinal route 4,5. The calculated half life in blood was 3 to 7 days, significantly shorter than the 45-day half-life of oral methyl mercury in adults. The objective of this study was to evaluate the pharmacokinetics of mercury in blood in a cohort of prematurely born infants with low birth weight who received a birth dose of hepatitis B vaccine containing thimerosal in concentrations still in use in vaccines provided through the World Health Organization (WHO) and to evaluate the excretion of mercury in these prematurely born infants by examining mercury levels in their stools and urine.
Methods
The study was conducted as a prospective observational study at the Hospital Durand (Buenos Aires, Argentina) from December, 2005 to October, 2006. Healthy infants with gestational ages of ≥ 32 and < 37 weeks were recruited, whose birth weight was ≥ 2000 but < 3000 grams; all infants were white Latinos. All children received exposure to thimerosal containing Hepatitis B vaccines as routinely administered in Argentina (described below), and all vaccines were administered by the investigators at the time of the enrollment visit. In each infant, samples of cord blood, urine, and stool were obtained before vaccination and at 1 time interval after vaccine, as described below. Each individual child contributed 1 pre-vaccination and 1 post-vaccination sample.
The study infants received the birth dose of HBV vaccine, which contained either 12.5 μg (Hepavax AGB; LG Chemicals, Iksan-City, Chun Buk, Korea) or 32.5 μg (Euvax B, Hepavax-Gene; LG Chemicals, Iksan-City, Chun Buk, Korea) of ethyl mercury. Infants were randomly assigned to have samples taken at 1 of the following time points after vaccination: 12 ± 3 hours, 24 ± 6 hours, 48 ± 8 hours, 5 ± 1 days, 11 ± 2 days, or 30 ± 3 days. This study was approved by the human subjects review boards of the University of Rochester and the R. Gutierrez Children’s Hospital and the Durand Hospital.
Laboratory Assessments
Cold-Vapor Atomic Fluorescence Spectrophotometry
Mercury levels in blood and urine were determined by cold-vapor atomic fluorescence spectrophotometry (CVAFS) using the Millennium Merlin/Galahad system (PA 10.035; P Analytical Ltd, Orpington, Kent, United Kingdom). The lower limit of detection in blood was 0.01 ng/mL, depending on sample volume4. CVAFS determinations measure total mercury, including ethyl or methyl mercury, and inorganic forms. The accuracy of the method for blood was assessed by using blood Seronorm 201605 (Seronorm, SERO, Billingstad, Norway) and for urine using urine Seronorm 20125 as the reference material. Total blood mercury determinations were performed in Rochester, NY, and all samples were coded and assayed by the laboratory without awareness of the vaccine cohort or order of sampling (before or after the vaccination).
Cold-Vapor Atomic Absorption
Mercury levels in stools were determined by cold-vapor atomic absorption. Mercury in positive stool samples was differentiated into total and inorganic mercury, and the difference between the 2 was assumed to be organic mercury. Stool mercury determinations were performed in Buenos Aires, Argentina; all sample were coded and assayed in a blinded manner. Results are reported in nanograms of total mercury per gram of stool dry weight.
Each lot of vaccine administered to the children in the study also was tested in Argentina for total mercury levels by using cold-vapor absorption. The organic mercury present in the vaccines was further speciated as ethyl or methyl mercury as described below.
Urinary γ-Glutamyl Transpeptidase and Creatinine Levels
Because mercury is known to be nephrotoxic, levels of γ-glutamyl transpeptidase (GTP) and creatinine were measured in urine samples collected during the study. GTP levels were determined by enzyme immunoassay. Urine creatinine levels were determined by using colorimetric analysis. Urine GCT levels were adjusted by measurement of urine creatinine to account for differing urine concentrations. These assays were performed in Argentina.
Statistical Methods
The primary objective of this study was to obtain an estimate of blood mercury levels at each time point after vaccination. A sample size of 10 per follow up sampling group produces a 95% CI equal to the sample mean ± 62 % of the SD. This study targeted enrollment to allow for 20% loss in follow up. Descriptive statistics were generated for levels of total blood, urine, and stool mercury at each time point.
Each child in this study had samples taken at a maximum of 2 time points, once before vaccination, and once at a randomly assigned time point after vaccination. We estimated the pharmacokinetics of mercury using a model that averages all of the samples obtained from the population rather than evaluating multiple samples from the same individual. Half-life estimates of blood mercury level were based on one-compartment first order pharmacokinetics model taking into account the dosage of thimerosal received and weight and age effects on volume and clearance. Specifically, the estimation process consisted of the following joint model (Model 1) being fit simultaneously:
CL/V is referred to as an elimination constant. For computational convenience, it is algebraically possible to reparameterize the elimination model in terms of a rate constant. The V is scaled as initial body weight (WT) and CL is scaled using allometric scaling of an exponent of 0.75. The median weight of 2.5 is used to standardize such scaling. 6-9 Therefore V and CL are further parameterized as below:
Based on the parameter estimates, the half time and the percent dose initially distributed to the blood compartment (assuming 8% of the body weight is blood) can be obtained using the following formula:
The variances of these estimates, hence the 95% confidence intervals (CI), are calculated using Delta method.
As an alternative, a different model (Model 2) explores the change of blood mercury level as the measurement of true concentration and eliminates the baseline term (C0) in the previous model, i.e.
Although this model is also plausible, it results in exclusion of 19 subjects who did not have either the pre-or post-vaccination results. Subjects whose post vaccination levels are lower than the pre-vaccination levels are assigned to have a level of 0 in this model. The calculations of the half time and percent dose distributed to the blood compartment follow the same formula.
All analyses were performed using SAS 9.1.3 (SAS Institute Inc, Cary, NC). A nonlinear model (Proc NLMIXED in SAS) was used in the model fitting.
Results
The demographics of the 72 prematurely-born infants enrolled in the study are shown in Supplementary Table I. Study children were an appropriate weight for gestational age at birth, and they lost an expected amount of weight in the first few days of life, with subsequent weight gain.
Some children were unable to contribute an adequate blood sample for mercury determination at the follow up visit. The numbers of blood, stool, and urine mercury determinations that were available from children at each visit are shown in Table II.
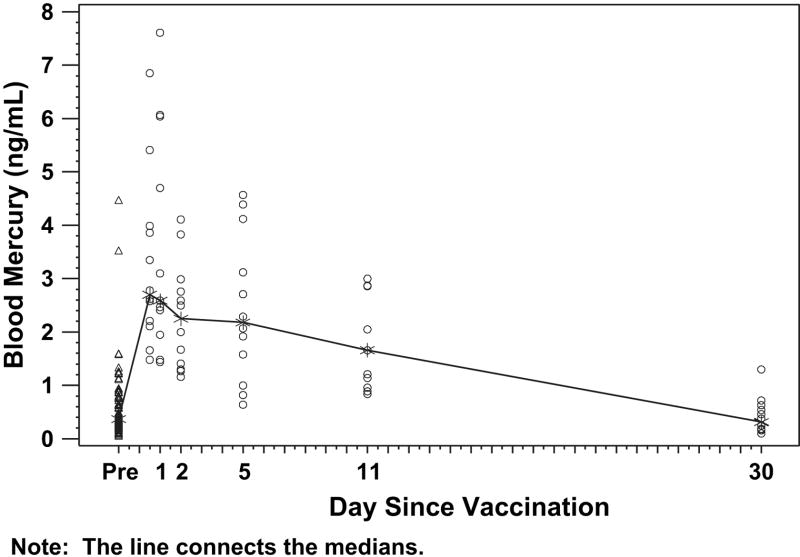
The density of mercury concentrations in blood, stool, and urine for the study group are presented in Figures 1 to 3. The highest levels of mercury in blood were detected in the first samples obtained after vaccination (ie, at 12-24 hours after vaccination). The highest level detected was 7.6 ng/mL in a newborn 24 hours after receiving a birth dose of HBV vaccine that contained 32.5 μg of mercury. Blood mercury levels correlated with the dose given in the vaccines, fell rapidly and had largely returned to baseline levels by day 10 after vaccination.
Figure 1.
Blood mercury levels before and after receipt of vaccines that contained thimerosal preservative. Infants received intramuscular vaccines and had blood sampling before vaccination (day 0) and were randomly assigned to have samples taken at a single time point after vaccination (see “Methods”). Each data point represents 1 observation. The median values for each time point are connected by the line.
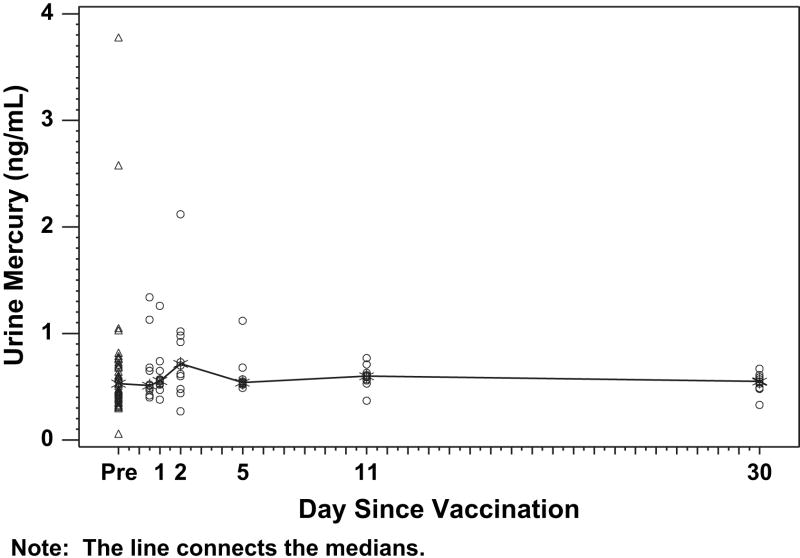
Figure 3.
Urinary mercury levels before and after receipt of vaccines that contained thimerosal preservative. Infants received intramuscular vaccines and had blood sampling before vaccination (day 0) and were randomly assigned to have samples taken at a single time point after vaccination (see “Methods”). Each data point represents 1 observation. The median values for each time point are connected by the line.
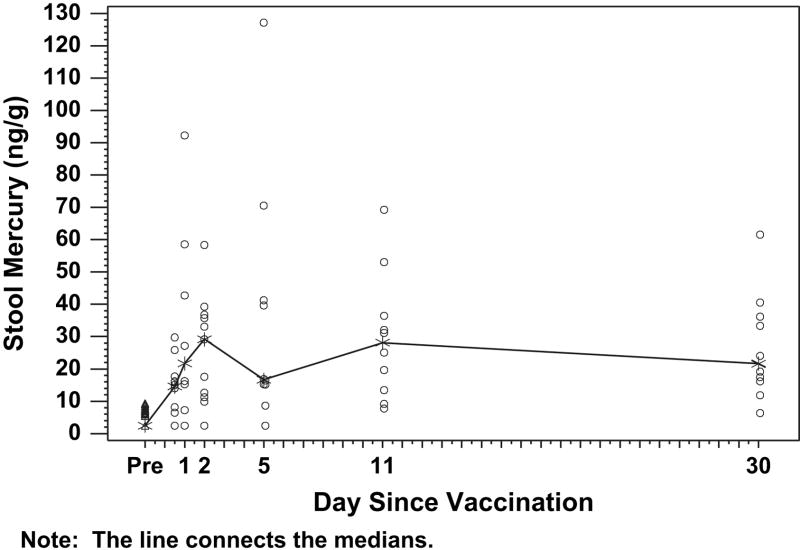
Mercury was detected in virtually all stool samples tested (Figure 2) and increased significantly after vaccinations. Mercury was detected in stools throughout the sampling time points. All of the mercury in stool samples was inorganic mercury. Mercury was nearly undetectable in urine in all samples (Figure 3). There was no evidence of clinically significant elevations of urine GGT and no association between blood mercury and urine GGT (data not shown).
Figure 2.
Stool mercury levels before and after receipt of vaccines that contained thimerosal preservative. Infants received intramuscular vaccines and had blood sampling before vaccination (day 0) and were randomly assigned to have samples taken at a single time point after vaccination (see “Methods”). Each data point represents 1 observation. The median values for each time point are connected by the line.
Blood mercury levels ranging from 0.1 to 4.5 ng/mL were detected in pre-vaccination samples from infants in this study. Therefore, methyl mercury must have been passed from the mother to these newborns transplacentally in very low amounts.
Half-life for blood mercury was estimated using two 1-compartment models as described in the “Methods.” The half-life of blood mercury was estimated to be 6.31 days (95% confidence interval 3.85-8.77 days) in model 1. In model 2, the half-life was estimated to be 4.97 days (95% confidence interval 2.58-7.37 days). The proportion of the initial dose from vaccination that was distributed to the blood compartment was estimated to be at 3% (with 95% confidence interval of 2.6% to 3.7%).
Discussion
We observed that blood mercury levels after intramuscular administration of thimerosal-containing vaccines to premature and low birth weight newborns were at their highest level shortly after vaccination and returned to pre-vaccination levels within about 10 days. Using a model that accounted for baseline mercury levels, ethyl mercury dosage, and timing of vaccination, we estimated that the blood half-life of mercury after administration of thimerosal was 4.97 to 6.3 days. Our estimate of mercury blood half-life after intramuscular ethyl mercury is consistent with recent comparison of the pharmacokinetics of mercury after intramuscular ethyl mercury in term newborns 5 and infant rhesus macaques. 10
The importance of blood levels of ethyl mercury for assessing toxicity is unknown, but blood levels have been shown to be a predictor of toxicity for methyl mercury exposure. 11,12 The low levels of mercury detected in this study suggest low risk for toxicity from this exposure.
There was an increase in stool mercury levels shortly after vaccination, which slowly fell afterward. This pattern is consistent with enterohepatic excretion similar to that described for methyl mercury; however these observations were made on spot (as opposed to 24-hour) collections, therefore, it is not possible to draw conclusions regarding the proportion of administered mercury that was eliminated in the stool and kinetics of the elimination process.
Because brain biopsies obviously could not be done, the use of the kidney (also a sensitive tissue to mercury toxicity) as a surrogate organ to assess toxicity seems reasonable. There was no evidence of significant urinary excretion of mercury in this study, similar to previous observations with methyl mercury and there was no damage to the kidney as evidenced by no significant changes in levels of urine GGT. The kidney, like the brain, is a sensitive issue to mercury toxicity.
Methyl mercury (CH3-Hg+) and ethyl mercury (CH3-CH2Hg+) have similar chemical structures, and these two short chain-chain alkyl mercurials generally have been assumed to possess similar toxicologic properties; however, much less information has been available for ethyl mercury, especially for humans. Animal data indicate that ethyl mercury, like methyl mercury, is readily transported to all tissues but has a shorter half-life10. Little is known about possible enterohepatic cycling and fecal excretion of ethyl mercury.
In 2000, Stajich et al reported measurements of total mercury levels before and after vaccinations with a hepatitis B vaccine that contained 12.5 μg of ethyl mercury in 15 preterm infants. 13 The premature infants by study design were ≤ 1000 grams (mean 748 grams); they received hepatitis B vaccine at birth. Mean pre-vaccination blood mercury level was 0.54 ± 0.79 ng/mL and the mean post-vaccination level was 7.36 ± 4.99 ng/mL, measured 48-72 hours after vaccination. These levels of mercury are clearly higher than those that we measured. However, an allometric adjustment based on differences between our study group’s mean weight of 2.4 kg and the Stajich et al mean weight of 0.7 kg allows the conclusion that the results are consistent and that expected blood mercury levels can be predicted based on birth weight and ethyl mercury dose (Table II).
This study has several limitations. Because methyl mercury was present in the blood of most study children prior to vaccination, our measurements of mercury post vaccination represent a mixture of ethyl mercury from the vaccines and methyl mercury from transplacental passage from mother to infant. Our measurements may therefore overestimate the actual amount of blood mercury contributed by ethyl mercury from vaccines. We were unable to determine the fate of the mercury after it leaves the blood, because our sampling was limited to blood, urine, and stool, therefore the data do not allow any conclusions about the proportion of the administered ethyl mercury that is ultimately excreted in the stools or the time course of that excretion, only that excretion occurs by the gastrointestinal route and that the kidneys do not seem to play an important role.
Our study provides data that may be useful in assessing the risks related to ethyl mercury exposure of prematurely born and low birth weight infants who receive thimerosal at dosages consistent with standard vaccination regimens. Concerns have been raised that administration of vaccines that contain thimerosal could cause an increased risk for autism. Epidemiologic studies have generally concluded that there is no evidence for such an association. 14-16 This study completes the assessment of the full range of thimerosal blood elimination kinetics from preterm infants who receive a birth dose of HBV vaccine, through infants to 6 months old 4,5. The results suggest that the risk assessment regarding exposure to thimerosal used as a preservative should be reevaluated in light of the demonstrated shorter blood half-life of ethyl mercury after vaccination.
Supplementary Material
Figure 4.
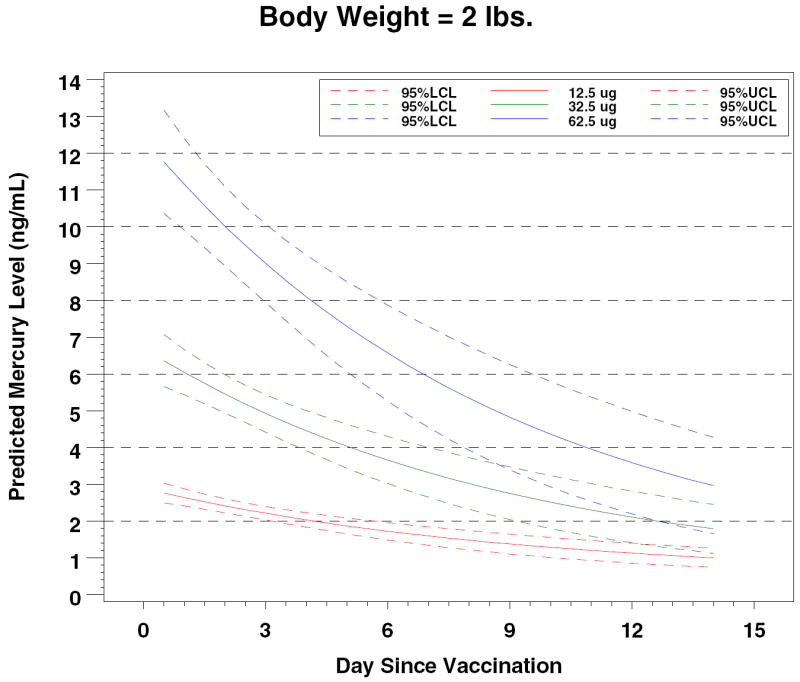
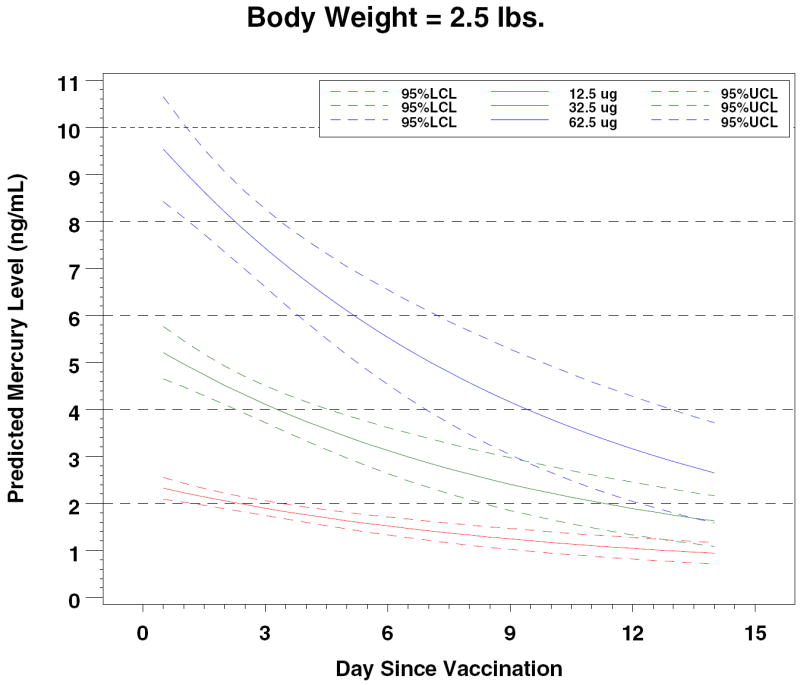
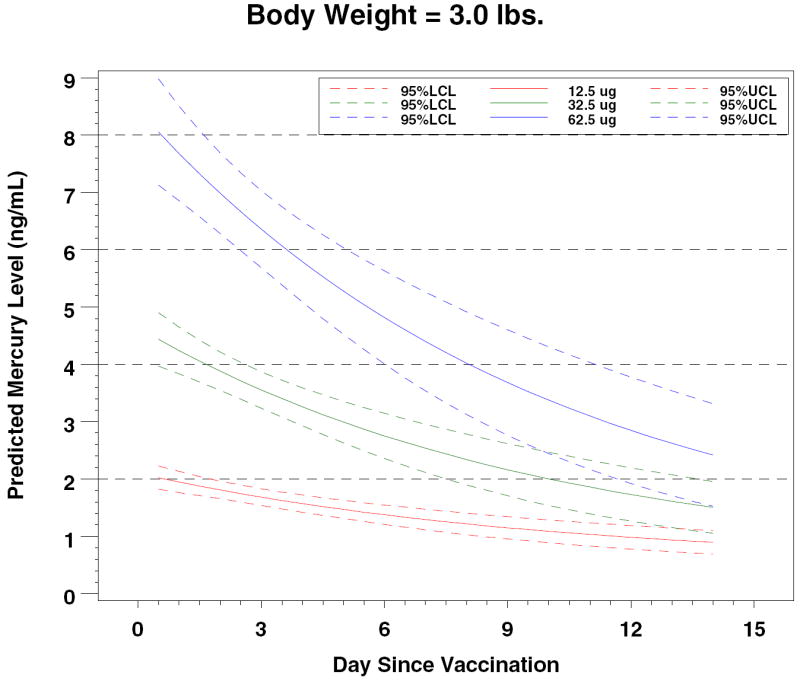
Predicted Mercury Levels by Dose and Body Weight
Table 1.
Summary of Mercury Levels in Blood, Urine and Stool
| Days Post Vac | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 5 | 11 | 30 | ||
| Body Weight (kg) | N | 72 | 12 | 11 | 12 | 12 | 11 | 12 |
| Mean | 2.4 | 2.3 | 2.3 | 2.6 | 2.2 | 2.3 | 3.2 | |
| SD | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | |
| Min | 1.8 | 2.1 | 2.0 | 2.0 | 1.8 | 1.9 | 2.4 | |
| Max | 3.0 | 2.6 | 2.8 | 2.9 | 2.7 | 2.9 | 3.8 | |
| Blood Hg (ng/mL) | N | 68 | 12 | 11 | 12 | 12 | 11 | 12 |
| Mean | 0.6 | 3.2 | 3.6 | 2.3 | 2.4 | 1.8 | 0.4 | |
| SD | 0.7 | 1.6 | 2.1 | 1.0 | 1.4 | 0.9 | 0.3 | |
| Min | 0.1 | 1.5 | 1.4 | 1.2 | 0.6 | 0.8 | 0.1 | |
| Q1 | 0.2 | 2.2 | 2.0 | 1.4 | 1.3 | 1.0 | 0.2 | |
| Median | 0.4 | 2.7 | 2.6 | 2.3 | 2.2 | 1.7 | 0.3 | |
| Q3 | 0.8 | 3.9 | 6.0 | 2.9 | 3.6 | 2.9 | 0.6 | |
| Max | 4.5 | 6.9 | 7.6 | 4.1 | 4.6 | 3.0 | 1.3 | |
| Urine Hg (ng/mL) | N | 54 | 11 | 10 | 12 | 9 | 11 | 11 |
| Mean | 0.6 | 0.6 | 0.6 | 0.8 | 0.6 | 0.6 | 0.5 | |
| SD | 0.5 | 0.3 | 0.2 | 0.5 | 0.2 | 0.1 | 0.1 | |
| Min | 0.1 | 0.4 | 0.4 | 0.3 | 0.5 | 0.4 | 0.3 | |
| Q1 | 0.4 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | |
| Median | 0.5 | 0.5 | 0.6 | 0.7 | 0.5 | 0.6 | 0.6 | |
| Q3 | 0.7 | 0.7 | 0.7 | 1.0 | 0.6 | 0.6 | 0.6 | |
| Max | 3.8 | 1.3 | 1.3 | 2.1 | 1.1 | 0.8 | 0.7 | |
Acknowledgments
We acknowledge the efforts of Margaret Langdon (University of Rochester) for assistance with mercury determinations; and Melinda Tibbals (EMMES Corporation) for study coordination and data management.
This study was supported by NIH NIAID contract AI 25460.
Footnotes
The authors declare no conflicts of interest.
References
- 1.American Academy of Pediatrics. Thimerosal in vaccines - An interim report to clinicians. Pediatrics. 1999;104:570–574. [PubMed] [Google Scholar]
- 2.Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in childhood vaccines. Pediatrics. 2001;107:1147–1154. doi: 10.1542/peds.107.5.1147. [DOI] [PubMed] [Google Scholar]
- 3.US Environmental Protection Agency. Mercury study report to congress. US Environmental Protection Agency; 1997. EPA-452/R-97-007. [Google Scholar]
- 4.Pichichero ME, Cernichiari E, Lopreiato J, Treanor J. Mercury concentrations and metabolism in infants receiving vaccines containing thimerosal: a descriptive study. Lancet. 2002;360:1737–1741. doi: 10.1016/S0140-6736(02)11682-5. [DOI] [PubMed] [Google Scholar]
- 5.Pichichero ME, Gentile A, Giglio N, Clarkson T, Zareba G. Mercury Levels in Newborns and Infants After Receipt of Thimerosal-Containing Vaccines. Pediatrics. 2008;121:208–214. doi: 10.1542/peds.2006-3363. [DOI] [PubMed] [Google Scholar]
- 6.Peters R. The ecological implications of body size. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 7.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–26. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 8.West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–9. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NHG. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology. 2002;96:1336–45. doi: 10.1097/00000542-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environmental Health Perspectives. 2005;113:1015–1021. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakir F, Damluji SF, Amin-Zaki L, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 12.Cox C, Clarkson TW, Marsh DO, AMin-Zaki L, Tikriti S, Myers CG. Dose response analysis of infants prenatally exposed to methyl mercury: an application of a single compartment model to single-strand hair analysis. Environ Res. 1989;49:318–332. doi: 10.1016/s0013-9351(89)80075-1. [DOI] [PubMed] [Google Scholar]
- 13.Stajich G, Lopez F, Harry S, Sexon W. Iatrogenic exposure to mercury after hepatitis B vaccination in preterm infants. The Journal of Pediatrics. 2000;136:679–81. doi: 10.1067/mpd.2000.105133. [DOI] [PubMed] [Google Scholar]
- 14.Parker S, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics. 2004;114:793–804. doi: 10.1542/peds.2004-0434. [DOI] [PubMed] [Google Scholar]
- 15.McCormick M, Bayer R, Berg A. Report of the Institute of Medicine: Immunization safety review: Vaccines and autism. National Academy Press; 2004. [Google Scholar]
- 16.Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: Prevalence and links with immunizations. Pediatrics. 2006;118:139–150. doi: 10.1542/peds.2005-2993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.