Abstract
Purpose
The purpose of this article is to detail a novel hypothesis regarding the role of changes in brain glucose delivery in menopausal hot flashes.
Organizing Framework
The impaired glucose delivery hypothesis of menopausal hot flashes is presented as a potential model of hot flash physiology. As foundational to the hypothesis, brain glucose physiology, specifically neurobarrier coupling, is presented in detail. With brain activation, glucose needs immediate increase; additional glucose is supplied through increased production of glucose transporter 1 (GLUT1) at the blood–brain barrier (BBB) and through vasodilation. Estrogen is important to this system in stimulating production of GLUT1. As estrogen declines at menopause, upregulation of GLUT1 is less efficient. As a consequence, neurobarrier coupling overcompensates with an excess neurovascular response, or a hot flash. Research supporting this hypothesis is briefly reviewed and new questions raised are reviewed.
Conclusions
The impaired glucose hypothesis of menopausal hot flashes proposes an inadequate neurobarrier response to neurometabolic stimulation as estrogen declines, resulting in additional neurometabolic stimulation with consequent neurovascular stimulation. In this model, the menopausal woman has diminished ability to respond to fluctuations in blood glucose over the course of the day, which results in hot flashes as a counter-regulatory response. This perspective accounts for observed physiological changes that have not been previously detailed. New research directions are identified.
Keywords: hot flashes, menopause, glucose transport, women's health
The hot flash, the most frequently reported symptom of menopause, has been associated with declining estrogen levels secondary to depletion of ovarian follicles in the aging ovary. However, a direct cause and effect relationship between estrogen level and hot flashes has not been demonstrated (Sterns & Hayes, 2002). In most studies, absolute concentrations of estrogen neither predict nor correlate with hot flash severity (Freedman, 2002; Hutton, Jacobs, Murray, & James, 1978). In addition, a threshold level of estrogen decline beyond which hot flashes are triggered has not been identified (Miller & Li, 2004). As cited in the report of the Panel on Assessing and Improving Measures of Hot Flashes (Miller & Li, 2004), estrogen withdrawal (not just deficiency) has a role in the hot flash experiences of menopausal women but “is not solely responsible for hot flashes because there appears to be no correlation between estrogen levels and vasomotor symptoms.” For any individual woman, it is likely that the relative decline in estrogen level plays a role in hot flashes. Relative changes in estrogen levels over time, and in relation to other metabolic factors, appear crucial in understanding estrogen's role in hot flash physiology.
Of the nearly 15 million women experiencing menopausal hot flashes in the United States, approximately 3 million seek medical assistance for symptom relief. The current management for hot flashes is hormone therapy (HT). But, recent reports of potential risks associated with the use of HT have created a dilemma for symptomatic women. One arm of the Women's Health Initiative Study was stopped when the 4th-year interim analysis indicated that HT users experienced significantly more invasive breast cancer than did placebo participants (Writing Group for the Women's Health Initiative Investigators, 2002). The researchers also noted that HT use was associated with increased risk of cardiovascular events, such as blood clots and heart attack. The Heart and Estrogen Replacement Study II (Grady et al., 2002) also found increased risk for cardiovascular events when HT was used as a preventive measure. Although most women in these studies were older and not experiencing menopausal symptoms, and despite the fact that the studies’ foci were on the preventive effects of HT rather than on menopause management, the findings have resulted in confusion and anxiety about HT and great caution in its use. Consequently, there is an urgent need to determine the physiologic mechanisms of hot flashes so that safe and effective management strategies can be developed. The purpose of this article is to present a novel hypothesis of hot flash physiology to guide future research in development of management strategies.
Impaired Glucose Delivery Hypothesis of Menopausal Hot Flashes
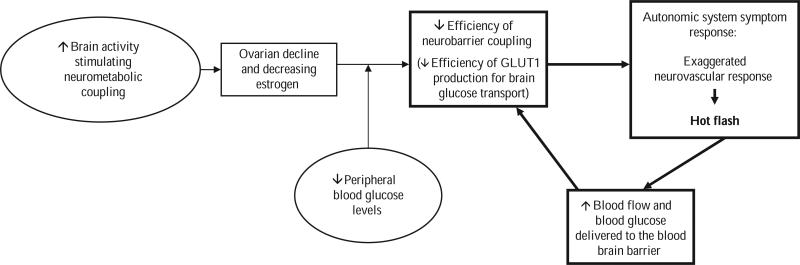
The impaired glucose delivery hypothesis of menopausal hot flashes proposes that hot flashes are related to changes in glucose delivery to the brain. The hypothesis (Figure 1) builds on the work of Dr. James W. Simpkins who has studied estrogen's effects on the brain. Rodent model studies in the basic research literature suggest that menopausal hot flashes result from transient inadequacies in central nervous system glucose transport (Shi & Simpkins, 1997). Additional observations in the rat model demonstrate that hot flashes (a tail flush) could be induced as blood glucose declined in ovarectomized female rats (Shi & Simpkins, 1997). Dr. Simpkin's laboratory found initial clinical support for the relationship of glucose to hot flashes in a small observational study in which three menopausal women were followed for 5 hr after they ate a standardized breakfast (Simpkins & Katovich, 1989). Hot flashes were absent when blood glucose was slightly elevated after eating but appeared when glucose concentrations fell to around 100 mg/dl between meals. Although the findings of this study are limited by the small sample, the lack of experimental manipulation of blood glucose levels, and the use of a digital thermistor as a gross measure of flashes, they suggested a potential relationship between glucose regulation and hot flash etiology.
Figure 1.
Model of the impaired glucose delivery hypothesis of menopausal hot flashes. GLUT1 = a glucose transporter.
This perspective was further refined through a review of the theoretical and empirical literature of two content areas: (a) brain glucose metabolism and (b) physiology of menopausal hot flashes. The literature provided detailed information about the central effects of the neuroendocrine changes associated with menopause. The central component of this hypothesis is that hot flashes are triggered as a result of an estrogen-related decline in glucose delivery to the brain. The decline of glucose transporter 1 (GLUT1), as mediated by declining estrogen, results in diminished ability to provide central glucose levels when needed during periods of increased glucose demand or decreased glucose supply. I propose that hot flashes can be stimulated when brain activity increases resulting in increased glucose consumption or decreased blood glucose. In either situation, GLUT1 is unable to quickly upregulate to maintain the supply. To better understand how impaired glucose delivery to the brain is related to menopausal hot flashes, it is important to first understand brain glucose physiology. Changes associated with menopause will then be identified to clarify the relationship among brain glucose regulation, menopause, and hot flashes.
Regulation of Glucose in the Brain
Glucose is the essential nutrient for brain glucose metabolism (Chih & Roberts, 2003) making rapid, efficient delivery of glucose to the brain crucial. However, only a 2-min supply is maintained. Any activation of the neurons increases energy demands. Simple activation of neurons from resting states generates both increased glucose consumption in the brain and increased transport at the blood–brain barrier (BBB; Leybaert, 2005) to meet neuron energy needs. Intensive neuron activation, such as in demanding mental activities or either cognitive or psychological stress, further increases glucose demand.
The BBB maintains separation between the blood plasma, with its glucose concentration of 5 mmol/L, and the brain extracellular space, with glucose concentration of 1 mmol/L (Leybaert, 2005). Given the differential of glucose concentrations, the movement of glucose molecules from plasma to the brain interstitium is mediated by facilitated diffusion by the carrier protein GLUT1 in the plasma membranes of endothelial cells (Pardridge, 1991). GLUT1 mediates brain glucose uptake from the blood supply at the BBB (Degroot & Jameson, 2001) through a neurobarrier coupling process which is proposed to be essential for hot flash physiology. This is one element of a dynamic process of brain glucose regulation which includes neurovascular, neurometabolic, and neurobarrier coupling (Leybaert, 2005).
Neuron activation initiates these three coupling processes that together provide the glucose needed to maintain neuron function. Neurometabolic coupling initiates the processes to provide the needed glucose. In the first step, glutamate is released by the neurons signaling glucose metabolism in the astrocytes (Magistretti & Pellerin, 1999). To support the increased metabolic needs related to this neuron activation, neurobarrier coupling is initiated (Leybaert, 2005). In this second coupling process, the GLUT1 at the BBB is stimulated to increase the number of molecules available to support the metabolic needs associated with neuronal activity. That is, when neuronal glucose concentrations are low, both the rate of transcription of GLUT1 messenger RNA (mRNA) and the number of GLUT1 molecules in the BBB increase in endothelial tissues. Cornford, Nguyen, and Landaw (2000) demonstrated that the increase in GLUT1 activity is evident within 3 min of neuronal activation. The final coupling process maintaining glucose levels in the brain is neurovascular coupling. In this process, neuron activity and related glucose needs cause relaxation of smooth muscle of the arterioles increasing blood vessel diameter and blood flow (Harder, Alkayed, Lange, Gebremedhin, & Roman, 1998; Iadecola, 1993; Kuschinsky, 1997; Lou, Edvinsson, & MacKenzie, 1987; Villringer & Dirnagl, 1995; Wahl & Schilling, 1993). This coupling, providing a functional hyperemia, meets the nutrient needs of brain cells.
In summary, with brain activation, astrocytes increase metabolism of glucose, the number of GLUT1 molecules increase at the BBB, and vasodilation provides increased surface area for GLUT1 transport and increased blood supply to provide molecules of glucose. The coupling processes together provide an adequate supply of glucose for neuronal functioning. The close relationship between neurovascular and neurobarrier coupling responses is mediated through the neurometabolic processes in the neurons and astrocytes. As demonstrated in brain slices from the rat cortex, activation of the neurons results in vasodilation through communication among neurons, astrocytes, and vascular beds (Zonta et al., 2003).
Three specific areas can affect the balance of the system supplying glucose to the neurons: brain activation, glucose transport, or glucose supply. Increasing cognitive or psychological stress activates increasingly more neurons, thus increasing glucose needs. Upregulation is needed in the transport/supply system to maintain homeostasis. However, the ability to increase glucose transport at the BBB as needed as well as having an adequate blood glucose supply to meet the demands are essential components of the system function. To understand the physiological basis of the hot flash, it is important to understand how menopause affects this balance.
The Role of Estrogen in Brain Glucose Regulation
Estrogen's role in this system is to facilitate the production of GLUT1 in endothelial tissues when energy demands increase. Estrogen augments GLUT1 in the cerebral cortex (Cheng, Cohen, Wang, & Bondy, 2001) enabling rapid response to changing glucose needs that are associated with brain activation by increasing the steady state levels of GLUT1 mRNA. It differentially regulates BBB permeability (Bake & Sohrabji, 2004) through stimulation of GLUT1 mRNA transcription in endothelial tissues. Estradiol's role in the transcription process is to upregulate glucose transporter expression; the presence of estradiol can increase glucose transport up to 40% in these tissues (Shi & Simpkins, 1997). Observations in the rat model demonstrate that declining estrogen concentrations in female rats reduce the transcription of GLUT1 mRNA (Shi & Simpkins, 1997). Shi and Simpkins (1997) demonstrated that, in ovarectomized rats, estrogen-deficient states lead to decreased production of GLUT1 and subsequent diminished glucose availability in neuroendothelial cells.
Peripheral Blood Glucose Effects on GLUT1 Activity
In an effort to maintain brain glucose levels, GLUT1 mRNA responds to glucose concentrations in the blood with downregulation during periods of increased glucose concentrations and with enhanced production in response to glucose decline (Rydzewski, Wozniak, & Raizada, 1991). When blood glucose is increased, such as after meals, brain glucose levels can be maintained by saturation of the available glucose molecules. However, when blood glucose levels are diminished, such as between meals, more GLUT1 molecules are needed to continue extraction of glucose from the blood and maintain brain glucose concentrations at a relatively constant state.
This relationship has been demonstrated in the rat model. Hot flashes can be induced in rodent models (as indicated by transient changes in tail color and temperature) using a variety of stimuli that reduce blood glucose or block the ability of brain cells to use glucose (Bishop & Simpkins, 1992, 1995; Namba & Sokoloff, 1984; Nehlig, Porrino, Crane, & Sokoloff, 1985; Simpkins, Andreadis, Millard, & Katovich, 1990). Conversely, blood glucose elevations preclude hot flash induction (Simpkins, Katovich, & Millard, 1990).
Before menopause, the system can compensate for declining blood glucose by rapid upregulation of GLUT1 transporter as needed. Such balance would be adequate to maintain the system unless there were a major decline in blood glucose. After menopause the system is much less effective in upregulating GLUT1 production, shifting the demand for a neurovascular response, or a hot flash. A close relationship between neurovascular and neurobarrier coupling responses is mediated through the neurometabolic processes in the neurons and astrocytes. As demonstrated in brain slices from the rat cortex, activation of the neurons results in vasodilation through communication among neurons, astrocytes, and vascular bed endothelium (Zonta et al., 2003). Activation of the coupling systems occurs within 3 s to meet the demand for increased glucose supply. The propositions of the impaired glucose hypothesis of menopausal hot flashes indicate an inadequate neurobarrier response to neurometabolic stimulation as estrogen declines, resulting in additional neurometabolic stimulation with consequent neurovascular stimulation.
Empirical Support
Subsequent well-controlled, adequately powered experimental studies provided further empirical support in development of this model. Dormire and Reame (2003) found that hot flash frequency varied with blood glucose level during a controlled experimental study. Participants were admitted to a clinical research center for close monitoring of blood glucose levels and skin conductance monitoring for hot flashes. Conditions of activity, room temperature, and dietary intake were controlled. The sample was composed of healthy postmenopausal women who agreed to withdraw from HT to participate in this study. On two sequential mornings, participants experienced randomly ordered periods of intravenous infusions of either glucose or normal saline following 11 hr of fasting. All participants received both infusions but were blinded as to infusion type. Continuous skin conductance monitoring for hot flashes and blood glucose monitoring per protocol were maintained. Between the experimental periods, monitoring of blood glucose and hot flashes continued with a nonfasting observation phase. The researchers found that hot flashes were reduced when blood glucose was elevated to between 130 and 140 mg/dl experimentally compared with fasting conditions with normal saline infusion (t = –2.4, df = 9, p = .04). In the experimental periods, only 2 hot flashes were experienced during glucose infusions whereas 23 were experienced during normal saline infusion.
Dormire and Howharn (2007) analyzed self-report data from a sample of 25 women as well as observational data from the study of Dormire and Reame (2003) with 12 women. In the self-report study, participants were symptomatic menopausal women who volunteered to maintain a 24-hr diary of both hot flashes and all nutrient intakes. During the observation period of the study of Dormire and Reame, study participants were fed the same foods at the same time and nutrient intake was closely calculated; both blood glucose and skin conductance were closely monitored.
Data analysis from these studies indicated that eating provided on average a 90-min hot flash free period. Also, as the time between meals increased, hot flash frequency was found to increase. According to the impaired glucose delivery hypothesis, the increased blood glucose levels provided by dietary intake suppress hot flash frequency whereas hot flashes emerge as blood glucose falls between meals.
Implications for Nursing Science and Nursing Research
This hypothesis provides significant insights into the nature of vasomotor symptoms. Examination of this hypothesis may guide nurses in the development of nonpharmacologic interventions aimed at blood glucose control through diet, meal schedule modification, and exercise (similar to that used in diabetic management) to manage hot flashes. Knowledge of hot flash physiology has the potential to contribute significantly to management of menopause and promoting health and quality of life for women during this transition.
At this point in the development of hot flash science, however, the hypothesis leads nurses to a variety of additional research opportunities. For instance, if hot flashes indicate inadequate glucose supply to the neurons, is the severity of menopausal hot flashes related to cognitive changes reported at menopause? A relationship between GLUT1 and Alzheimer's disease (AD) is indicated in research comparing autopsy tissue of healthy brain and that of persons with AD. In their work, Simpson, Chundu, Davies-Hill, Honer, and Davies (1994) demonstrated that there is a reduced concentration of GLUT1 in tissues of persons with AD. Intriguingly, the deficit remains even with correction for neuronal loss suggesting that GLUT1 change may be directly related to the pathology of AD. Should the altered glucose delivery hypothesis of hot flashes be supported, it would suggest a mechanism for cognitive change.
Some questions focus on the relationship of blood glucose changes and hot flashes: Is rate of change of glucose a signal for hot flash events? Is there a set point glucose level that signals hot flash events? What is the brain detecting to initiate hot flash responses (absolute level of glucose or rate of change)?
Questions related to the effects of other factors as stimuli or treatments are also raised. For instance, does estrogen change the hot flash compensatory response set point (threshold) or rate of glucose change or absolute level of blood glucose or all? Is there evidence of brain hypometabolism that precipitates flashes? Do women with flashes have higher brain activity than women without flashes? Do factors that decrease hot flashes do so by increasing peripheral glucose or by decreasing brain glucose metabolism?
The effects of hot flashes on other aspects of menopause also remain to be explored. What are the effects of the menopause transition on memory? What are the effects of hot flashes on memory? Why do hot flashes cease after a period of time for most women?
Based on the model and the physiology of diabetes, questions regarding the menopausal hot flash experience of women with diabetes arise. Does the diabetic woman's experience of hot flashes differ from that of the nondiabetic woman in the menopause transition? Is it decreasing glucose or increasing insulin that triggers hot flashes? Examination of these questions will contribute significantly to nursing science in the care and health promotion of women during the menopause transition.
Conclusion
The complex nature of menopausal hot flashes necessitates examination of new perspectives to explain the phenomenon. The novel hypothesis presented in this article directs such consideration. This hypothesis indicates that estrogen decline at menopause results in an inadequate GLUT1 neurobarrier response to the glucose needs of neurons. Consequently, there is an exaggerated neurovascular response, or a hot flash, as a counter-regulatory response. This perspective of a brain-focused process is supported by findings from clinical studies in which peripheral blood glucose was manipulated.
Examination of a new paradigm leads nurse researchers to new directions in health promotion for women at midlife. This hypothesis should direct development of nonpharmacologic management strategies for women who suffer from hot flashes. Furthermore, this novel hypothesis raises significant and intriguing questions for researchers and clinicians focused on women's health.
Acknowledgment
This study was supported by NIH grants R15NR009023-01A2, M01-RR00042, 5T32NR07074-08, and 5-R01-AG15083, the Southern Nursing Research Society Small Grants Award, and the University of Texas at Austin Special Research Grant.
References
- Bake S, Sohrabji F. 17β-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Bishop J, Simpkins JW. Role of estrogens in peripheral and cerebral glucose utilization. Review of Neurosciences. 1992;3:121–137. doi: 10.1515/REVNEURO.1992.3.2.121. [DOI] [PubMed] [Google Scholar]
- Bishop J, Simpkins JW. Estradiol enhances brain glucose uptake in ovariectomized rats. Brain Research Bulletin. 1995;36:315–320. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. The FASEB Journal. 2001;15:907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- Chih CP, Roberts EL. Energy substrates for neurons during neural activity: A critical review of the astrocyte-neuron lactate shuttle hypothesis. Journal of Cerebral Blood Flow & Metabolism. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. American Journal of Physiology. 2000;79:H1346–H1354. doi: 10.1152/ajpheart.2000.279.3.H1346. [DOI] [PubMed] [Google Scholar]
- DeGroot LJ, Jameson JL, editors. Endocrinology. 4th ed. W.B. Saunders Company; Philadelphia: 2001. [Google Scholar]
- Dormire SL, Howharn C. The effect of dietary intake on hot flashes at menopause. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2007;36:255–262. doi: 10.1111/j.1552-6909.2007.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormire SL, Reame N. Menopausal hot flash frequency changes in response to experimental manipulation of blood glucose. Nursing Research. 2003;52:338–343. doi: 10.1097/00006199-200309000-00008. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Hot flash trends and mechanisms. Menopause. 2002;9:151–152. doi: 10.1097/00042192-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatkey M, et al. for the HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (HERS II). JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: Hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- Hutton JD, Jacobs HS, Murray MA, James VHT. Relation between plasma estrone and estradiol and climacteric symptoms. Lancet. 1978;1:671–627. doi: 10.1016/s0140-6736(78)90796-1. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: Is nitric oxide the missing link? Trends in Neuroscience. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W. Neuronal-vascular coupling. A unifying hypothesis. Advances in Experimental Medical Biology. 1997;413:167–176. [PubMed] [Google Scholar]
- Leybaert L. Neurobarrier coupling in the brain: A partner of neurovascular and neurometabolic coupling? Journal of Cerebral Blood Flow & Metabolism. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- Lou HC, Edvinsson L, MacKenzie ET. The concept of coupling blood flow to brain function: Revision required? Annals of Neurology. 1987;22:289–297. doi: 10.1002/ana.410220302. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philosophy of Trans R Soc Lond B Biological Sciences. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HG, Li RM. Measuring hot flashes: Summary of a National Institutes of Health Workshop. Conference Report. Mayo Clinic Proceedings. 2004;79:777–781. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- Namba H, Sokoloff L. Acute administration of high doses of estrogen increases glucose utilization throughout the brain. Brain Research. 1984;291:391–394. doi: 10.1016/0006-8993(84)91276-9. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Porrino LJ, Crane AM, Sokoloff L. Local cerebral glucose utilization in normal female rats: variations during the estrous cycle and comparisons with males. Journal of Cerebral Blood Flow and Metabolism. 1985;5:393–400. doi: 10.1038/jcbfm.1985.54. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Advances in cell biology of blood-brain barrier transport. Seminars in Cell Biology. 1991;2:419–426. [PubMed] [Google Scholar]
- Rydzewski BZ, Wozniak MM, Raizada MK. Glucose transporters in central nervous system glucose homeostasis. Advances in Experimental Medicine & Biology. 1991;293:397–404. doi: 10.1007/978-1-4684-5949-4_35. [DOI] [PubMed] [Google Scholar]
- Shi J, Simpkins JW. 17 β-Estradiol modulation of glucose transporter 1 (GLUT1) expression in blood-brain barrier. American Journal of Physiology: Endocrinology and Metabolism. 1997;272:E1016–E1022. doi: 10.1152/ajpendo.1997.272.6.E1016. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's Disease. Annals of Neurology. 1994;35(5):546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Andreadis DK, Millard WJ, Katovich MJ. The effect of cellular glucoprivation and skin temperature regulation in the rat. Life Science. 1990;47:107–115. doi: 10.1016/0024-3205(90)90223-e. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Katovich MJ. Relationship between blood glucose and hot flushes in women and an animal model. In: Lomax P, Schönbaum E, editors. Thermoregulation: Research and clinical applications. Karger; Basel, Switzerland: 1989. pp. 95–100. [Google Scholar]
- Simpkins JW, Katovich MJ, Millard WJ. Glucose modulation of skin temperature responses during morphine withdrawal in the rat. Psychopharmacology. 1990;102:213–220. doi: 10.1007/BF02245924. [DOI] [PubMed] [Google Scholar]
- Stearns V, Hayes DF. Cooling off hot flashes. Journal of Clinical Oncology. 2002;20:1436–1438. doi: 10.1200/JCO.2002.20.6.1436. [DOI] [PubMed] [Google Scholar]
- Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovascular Brain Metabolism Reviews. 1995;7:240–276. [PubMed] [Google Scholar]
- Wahl M, Schilling L. Mediators inducing stasis in cerebral microvessels during ischemia. In: Tomita M, McHedlishvili G, Rosenblum W, Heiss WD, editors. Microcircula-tory Stasis in the Brain. Elsevier; New York: 1993. pp. 305–310. [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. Journal of Physiology. 2003;553(Pt 2):407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]