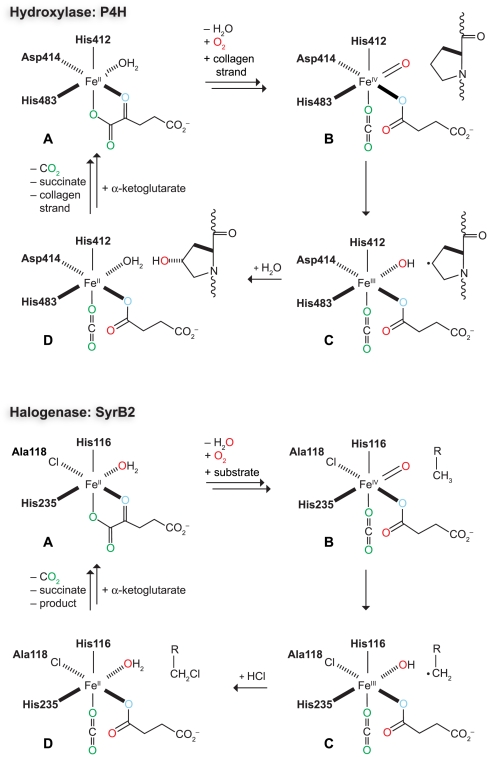
Figure 3. Proposed reaction mechanisms for the hydroxylase P4H (top) and halogenase SyrB2 (bottom).
In both mechanisms, the intermediates are labeled A–D. A Iron(II) in the active site is bound by a 2-His–1-Asp motif in P4H, but a 2-His–1-chloride in SyrB2. The configuration of these residues is not known for human P4H, but is drawn in analogy to SyrB2 (Figure 2B). B The reactive iron(IV)-oxo species is formed upon decarboxylation of α-ketoglutarate. C The iron(IV)-oxo species abstracts a hydrogen atom from the substrate producing a radical intermediate. D In the hydroxylase reaction, the substrate radical recombines with the hydroxyl group. In the halogenase reaction, the substrate radical reacts with the chloride ligand.