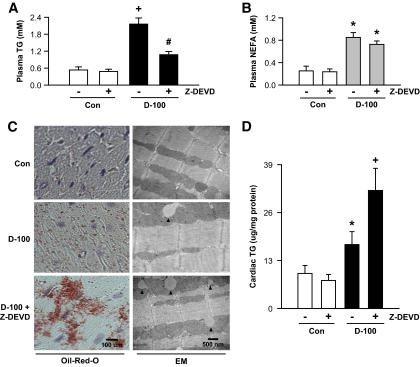
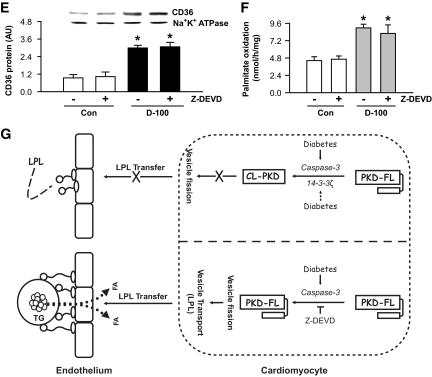
FIG. 7.
Inhibition of caspase-3 facilitates a striking increase in triglyceride accumulation in d-100 hearts. Plasma collected at termination was evaluated for triglyceride (A) and NEFA (B) using diagnostic kits. Oil Red O staining of representative cardiac sections showing lipid accumulation is described in C (left panel). Bar = 100 μm. Representative electron micrograph of hearts from different groups is depicted in C (right panel). Bar = 500 nm. Black arrow head, lipid-like vacuoles. Cardiac triglyceride was extracted with organic solvent and determined using high-performance liquid chromatography (D). Plasma membranes were isolated and used for identification of CD36 (E). Palmitate oxidation in myocytes isolated from the different groups was assessed by measuring 14CO2 (F). Results are the means ± SE of 3–5 rats in each group. *Significantly different from control subjects; +significantly different from all other groups; #significantly different from untreated d-100, P < 0.05. A graphic representation of caspase-3 control of cardiac metabolism is described in G. After severe hypoinsulinemia, a decrease in full-length PKD requires both activation of caspase-3 together with loss of the binding protein 14-3-3ζ. Proteolysis of PKD is unable to transfer cardiac LPL to the vascular lumen. The caspase inhibitor, Z-DEVD, effectively lowers caspase-3 activity in d-100 hearts, prevents PKD cleavage, and increases LPL vesicle formation and translocation to the coronary lumen. This increase in luminal LPL is associated with a decline in circulating triglyceride but a striking cardiac triglyceride accumulation. AU, arbitrary unit; TG, triglyceride; Casp3, caspase-3. (A high-quality color digital representation of this figure is available in the online issue.)