Abstract
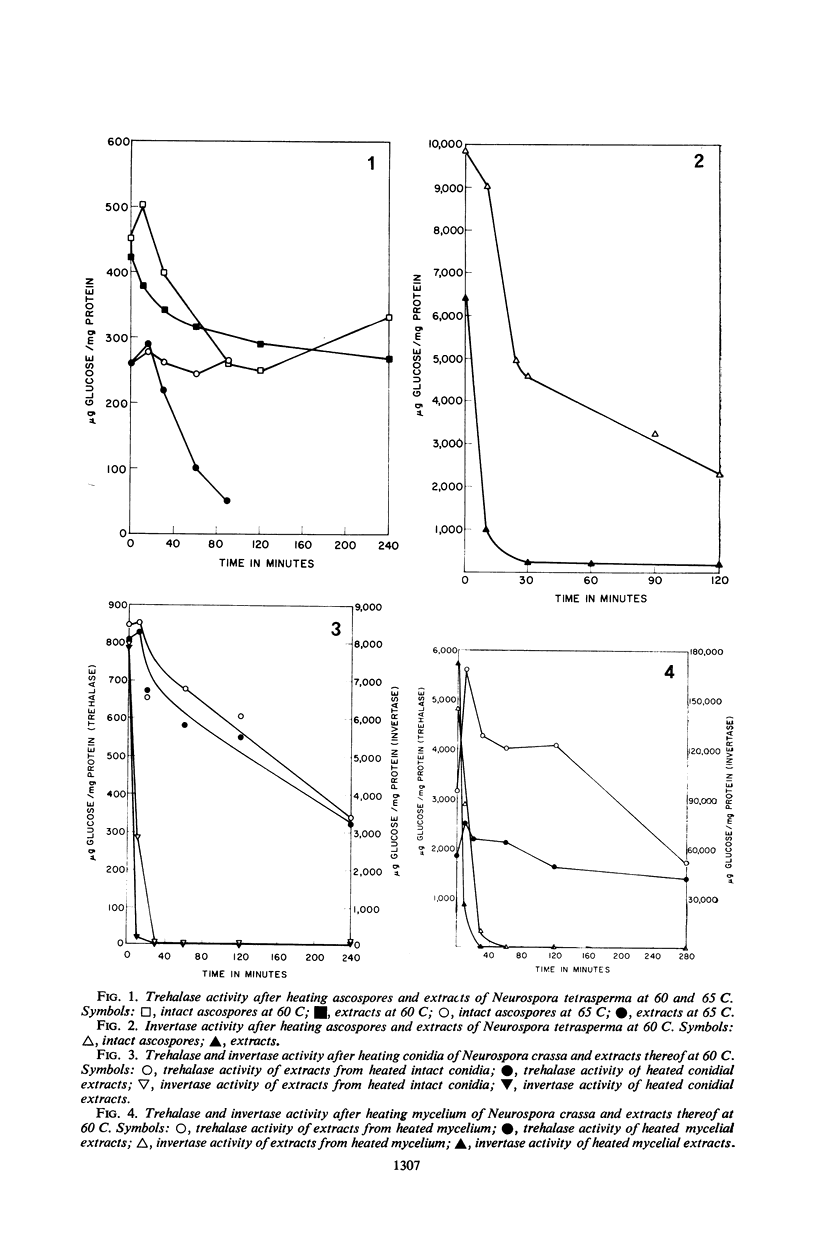
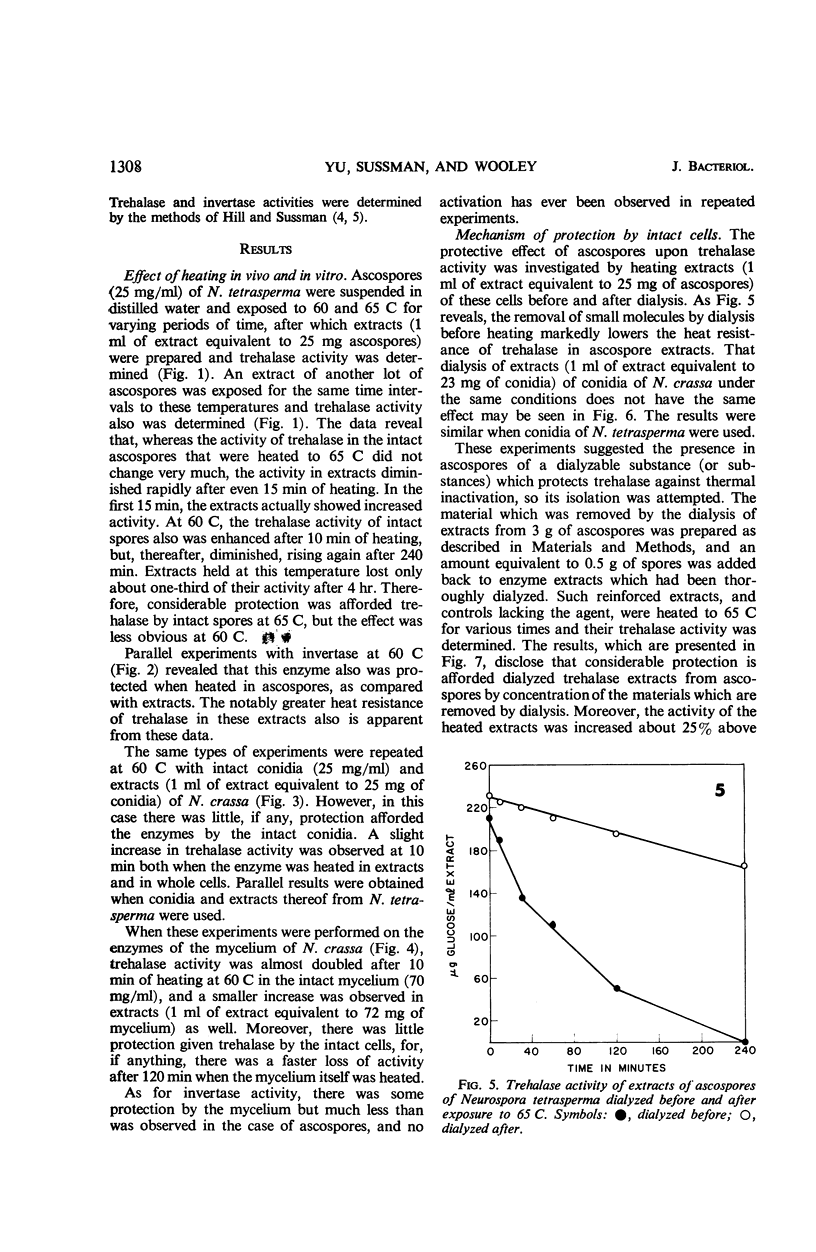
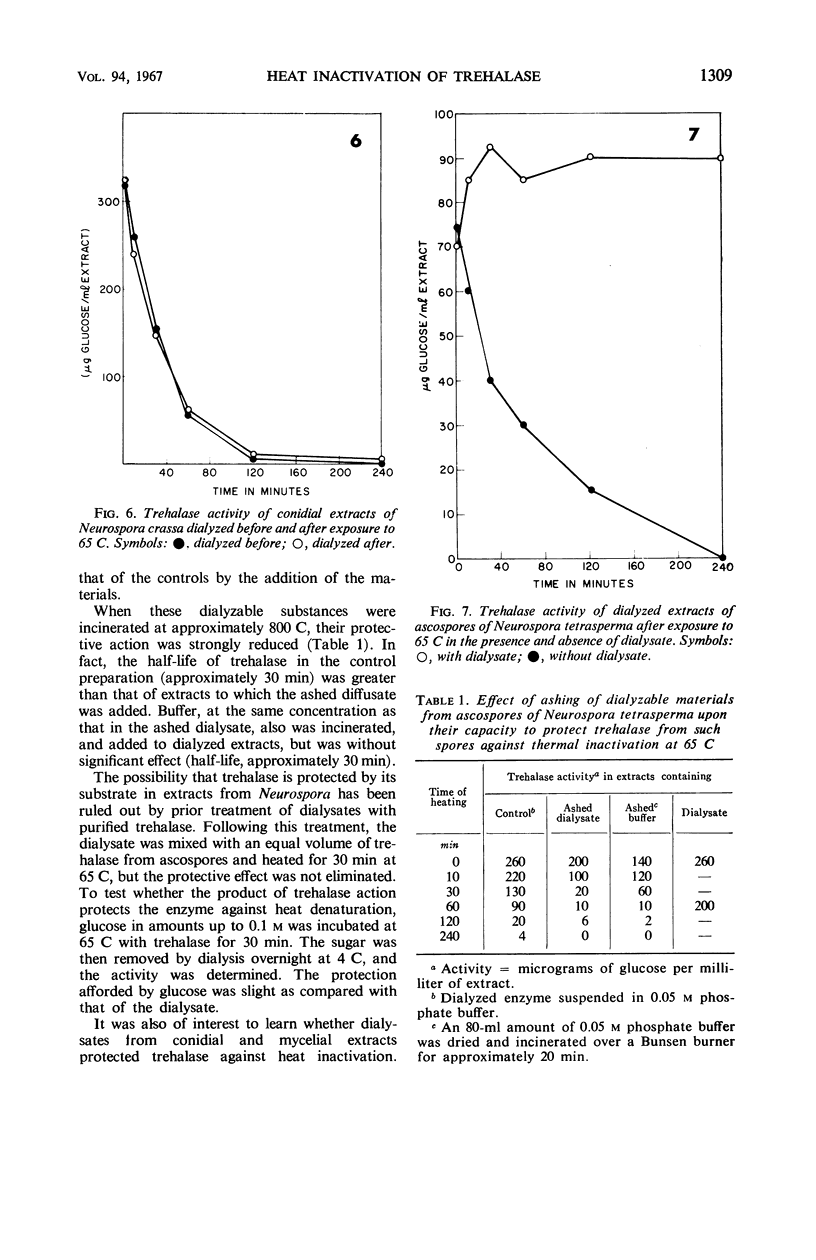
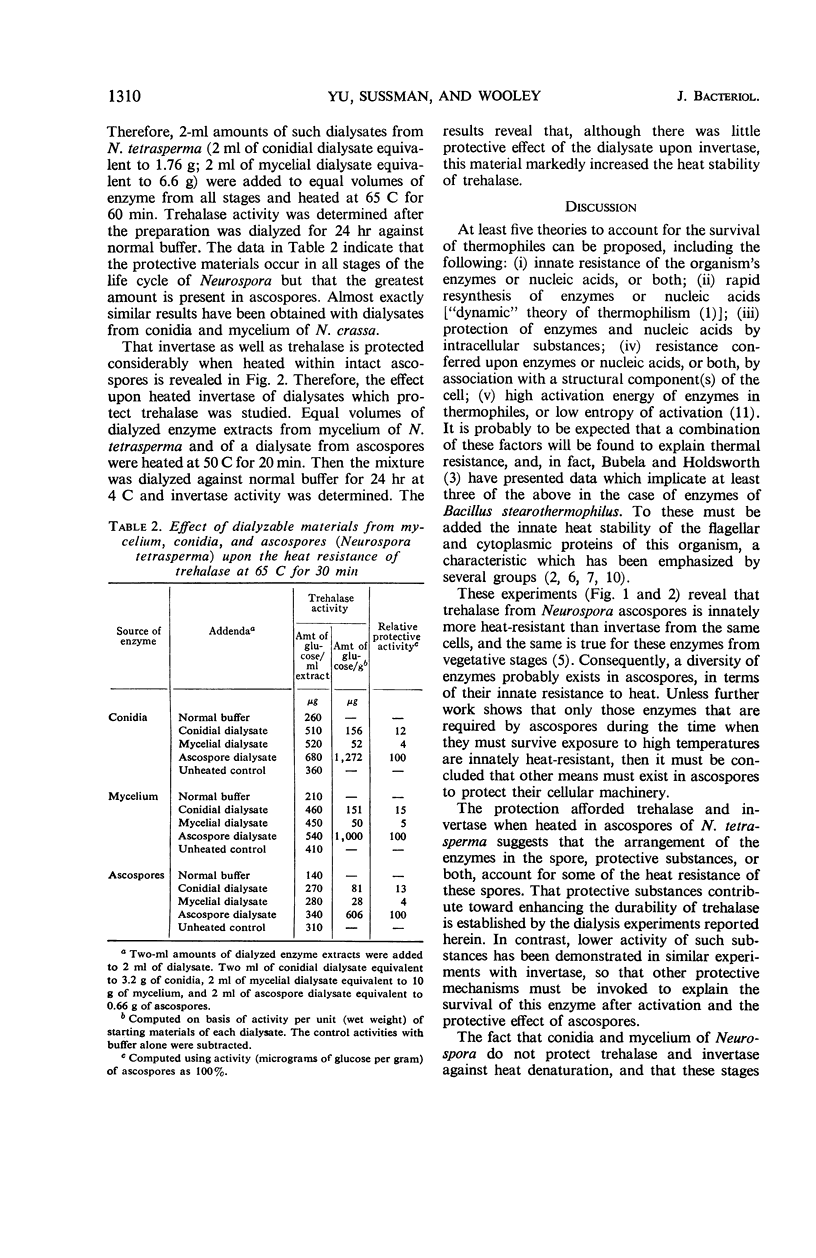
The half-life of trehalase and invertase at 65 and 60 C was found to be much greater when intact ascospores of Neurospora tetrasperma were heated, as compared with extracts. By contrast, no protection was afforded these enzymes when they were heated in intact conidia and mycelium of N. crassa or N. tetrasperma. The protective effect of ascospores for trehalase was further investigated by heating ascospore extracts before and after dialysis. The removal of small molecules by dialysis lowered the heat resistance of trehalase significantly in such extracts. When the dialysate from extracts of mycelium, conidia, or ascospores was added to dialyzed enzyme extracts, that from ascospores was by far the most active. However, the same dialysates had only a small protective effect on invertase. The addition of ashed dialysates did not protect trehalase, and trehalose and glucose protected less effectively than the dialysate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. The thermophilic aerobic sporeforming bacteria. Bacteriol Rev. 1953 Jun;17(2):125–173. doi: 10.1128/br.17.2.125-173.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelunxen R. E. Crystallization of thermostable glyceraldehyde-3-phosphate dehydrogenase from Bacillus stearothermophilus. Biochim Biophys Acta. 1966 Aug 10;122(2):175–181. doi: 10.1016/0926-6593(66)90059-2. [DOI] [PubMed] [Google Scholar]
- Bubela B., Holdsworth E. S. Protein synthesis in Bacillus stearothermophilus. Biochim Biophys Acta. 1966 Aug 17;123(2):376–389. doi: 10.1016/0005-2787(66)90290-5. [DOI] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. PURIFICATION AND PROPERTIES OF TREHALASE (S) FROM NEUROSPORA. Arch Biochem Biophys. 1963 Sep;102:389–396. doi: 10.1016/0003-9861(63)90246-7. [DOI] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler H., Mallett G. E., Adye J. MOLECULAR BASIS OF BIOLOGICAL STABILITY TO HIGH TEMPERATURES. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):464–477. doi: 10.1073/pnas.43.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANNING G. B., CAMPBELL L. L. Thermostable alpha-amylase of Bacillus stearothermophilus. I. Crystallization and some general properties. J Biol Chem. 1961 Nov;236:2952–2957. [PubMed] [Google Scholar]
- MARSH C., MILITZER W. Thermal enzymes. VIII. Properties of a heat-stable inorganic pyrophosphatase. Arch Biochem Biophys. 1956 Feb;60(2):439–451. doi: 10.1016/0003-9861(56)90449-0. [DOI] [PubMed] [Google Scholar]
- SUSSMAN A. S. Changes in the permeability of ascospores of Neurospora tetra-sperma during germination. J Gen Physiol. 1954 Sep 20;38(1):59–77. doi: 10.1085/jgp.38.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSSMAN A. S. The role of trehalose in the activation of dormant ascospores of neurospora. Q Rev Biol. 1961 Jun;36:109–116. doi: 10.1086/403332. [DOI] [PubMed] [Google Scholar]


