Table 1.
One-pot synthesis of indoles under Sonogashira coupling conditions.a
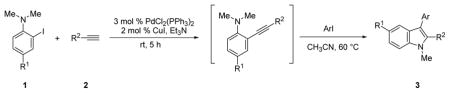 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | R1 | R2 | Ar | time (h) |
3 | % yieldb | |
| Step 1 | Step 2 | |||||||
| 1 | 1a | H | C6H5 | 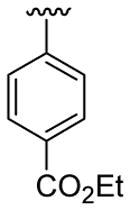 |
5 | 4 | 3a | 82 |
| 2 | 1b | Br | 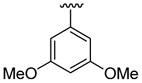 |
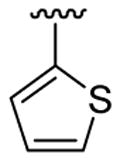 |
5 | 12 | 3j | 83 |
Representative procedure: Step 1) 2-Iodoaniline 1 (0.500 mmol), terminal alkyne 2 (0.525 mmol), PdCl2(PPh3)2 (0.015 mmol), CuI (0.010 mmol), and 3 mL of Et3N were mixed in a sealed 4-dram vial. The reaction was stirred at room temperature for the indicated time. Step 2) Aryl iodide (0.550 mmol) and 3 mL of CH3CN were added to the reaction mixture of Step 1. The resulting mixture was stirred at 60 °C for the indicated time.
Isolated yields of indole product after column chromatography.
