Figure 1.
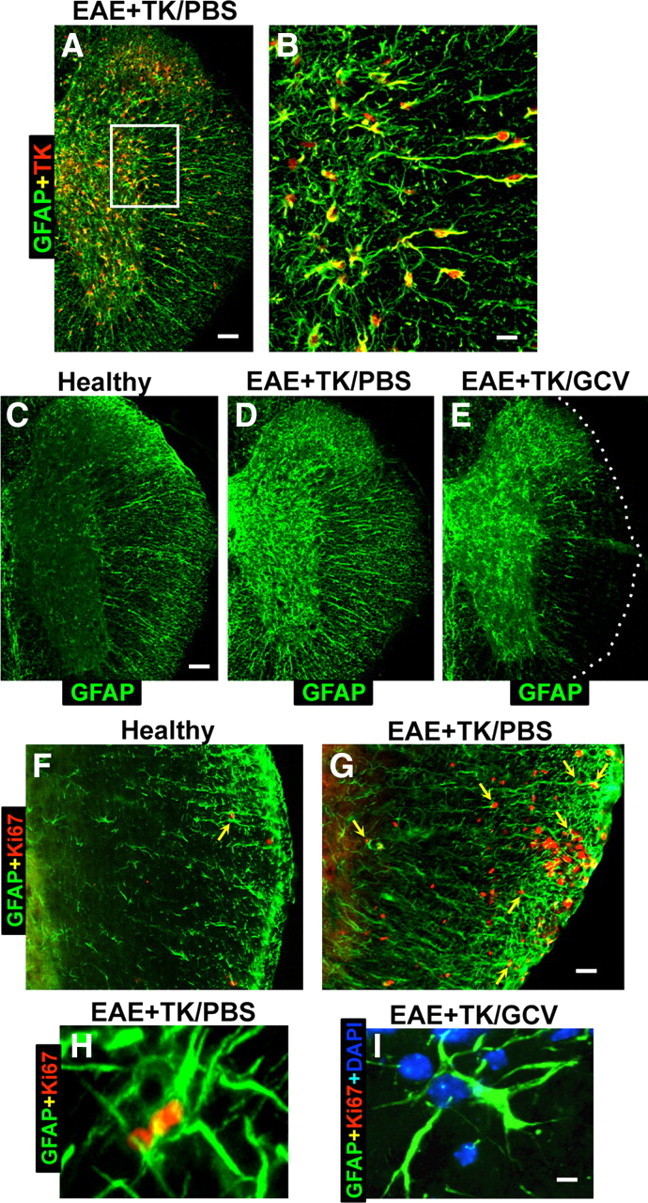
Verification of strategy for transgenic ablation of proliferating astrocytes during EAE. A–I, Single- or two-color immunofluorescence for GFAP (green, A–I) and either transgene derived-TK (red, A–E) or the cell cycle marker, Ki67 (red, F–I) in mouse spinal cord. A, B, Survey and detail images show that in EAE-induced GFAP-TK transgenic mice treated with PBS (EAE+TK/PBS), all TK-expressing cells also express GFAP. Note that TK staining is prominent in astrocyte cell bodies, whereas GFAP is distributed primarily in cell processes. C–E, Survey images of thoracic spinal cord from GFAP-TK mice that were healthy (C) or induced with EAE and given either PBS (D) or GCV (E) to ablate proliferating, transgene-expressing astrocytes (EAE+TK/GCV). Note that GFAP staining is increased in EAE+TK/PBS (D) relative to healthy (C) and reduced in a patchy manner in EAE+TK/GCV (E) relative to EAE+TK/PBS (D), particularly in white matter. F, G, Survey images show that there are few proliferating, Ki67-positive cells in a healthy mouse and that spontaneously proliferating astrocytes (arrow) are rare (F), whereas in a mouse with EAE+TK/PBS, there are many proliferating, Ki67-positive cells, including proliferating astrocytes (arrows) (H). H, I, Detail images show proliferating, Ki67-positive, perivascular astrocytes in a mouse with EAE+TK/PBS (H) and the persistence of surviving, nonproliferating perivascular astrocytes in a mouse with EAE+TK/GCV (I). Scale bars: A, 100 μm; B, 25 μm; C–E, 125 μm; F, G, 50 μm; H, I, 4 μm.
