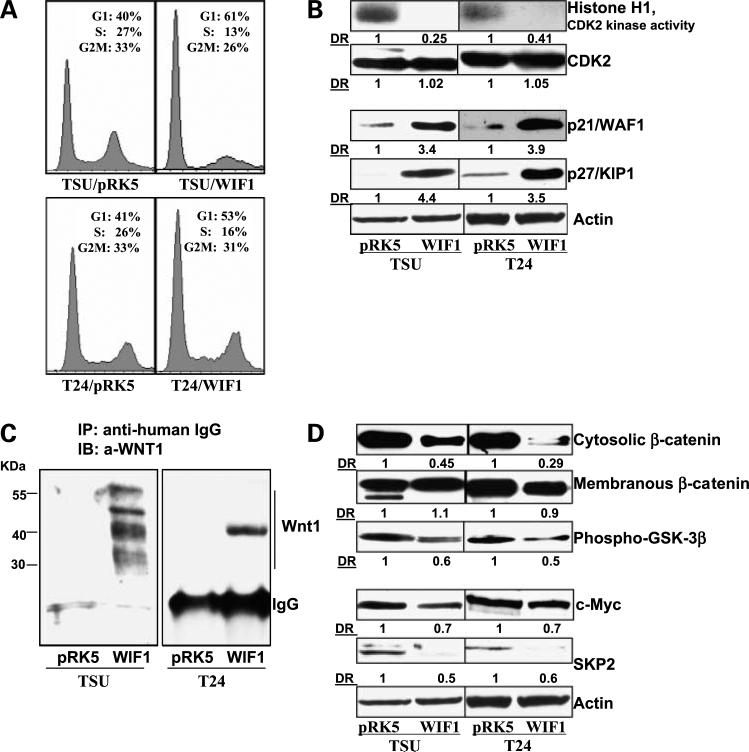
Figure 2.
WIF1 induces G1 arrest and inhibits the Wnt/β-catenin-mediated signaling pathway in T24 and TSU-PR1 cells. A, cell cycle distribution was analyzed by flow cytometry as detailed in Materials and Methods. Graphical presentation of percent cell cycle distribution in T24 and TSU-PR1 cells expressing pRK5 control or WIF1, respectively. Mean of three samples for each experiment. Results were similar in two independent experiments. B, histone H1-associated CDK2 kinase activity was determined in T24 and TSU-PR1 cells expressingpRK5 control or WIF1, respectively, as described in Materials and Methods. Western blottinganalysis of p27/Kip1, p21/WAF1, and CDK2 in T24 and TSU-PR1 cells expressingpRK5 control or WIF1, respectively. Total protein lysates were used for SDS-PAGE and Western blotting. Membranes were detected by labeling with primary antibodies followed by addition of peroxidase-conjugated secondary antibody and visualized by ECL. The membrane was “stripped” and reprobed with anti-actin antibody for determination of protein loading. Representative of three independent experiments. C, immunoprecipitation assay of WIF1/Wnt1 complex. Concentrated conditioned medium from culturing T24 and TSU-PR1 cells expressing pRK5 control or WIF1 were incubated with anti-human IgG and protein A-agarose beads overnight at 4°C and probed with anti-Wnt1 antibody. Representative of three independent experiments in each case. D, Western blottinganalysis of β-catenin, phospho-GSK3β, c-myc, and SKP2 protein expression in T24 and TSU-PR1 cells expressing pRK5 control or WIF1, respectively. Membranes were “stripped” and reprobed with anti-actin antibody for determination of protein loadingcontrol. Representative of three independent experiments. DR, densitometry measurement ratio relative to control treatment after adjusting for densities of β-actin loading control.