Figure 1. Net transport of lipid droplets requires Kinesin-1.
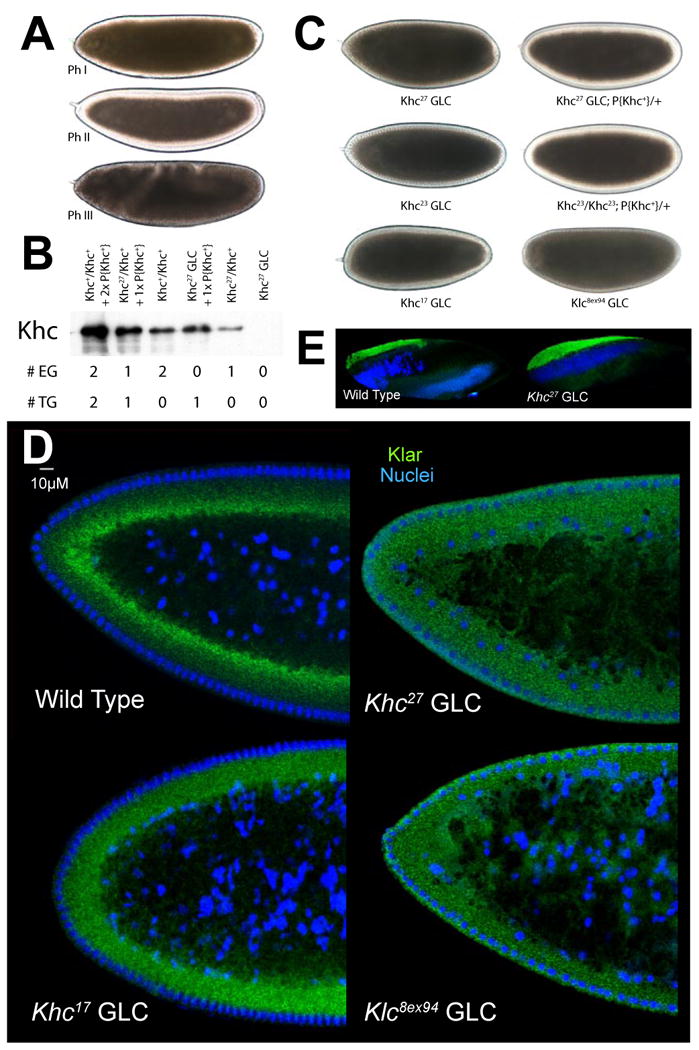
(A) Lipid-droplet distribution in wild-type embryos, as revealed by transparency of the embryo periphery in transmitted light. During Phases I (top) and III (bottom), the periphery is opaque because lipid droplets are present throughout. In Phase II (middle), droplets move inward and as a result the periphery is transparent (“clearing”).
(B) Khc levels in embryos of various genotypes. Top: full description of genotypes; bottom: summary how many copies of the endogenous Khc gene (#EG) and the Khc transgene P{Khc+} (#TG) are present. Proteins were extracted from embryos laid by mothers of the indicated genotypes, and Khc was detected by Western analysis. Coomassie Blue staining of membranes demonstrated equal protein loading across lanes (not shown).
(C) Kinesin-1 function is required for clearing of the embryonic periphery in Phase II. When embryos either completely lack one of the Kinesin-1 subunits (Khc27 GLC and Klc8ex94 GLC) or contain only mutant Kinesin (Khc23 GLC and Khc17 GLC), they fail to undergo clearing during Phase II. Expression of wild-type Khc (from the P{Khc+} transgene) restores clearing in the absence of endogenous Khc (Khc27 GLCs) or when all endogenous Khc is mutant (embryos from mothers homozygous for Khc23, rescued to viability by P{Khc+}).
(D) Kinesin-1 function is required for net inward transport of lipid droplets in Phase II. Embryos of various genotypes were stained for DNA (blue) and the droplet-marker Klar (green). In the wild type, Klar accumulates basally, away from the peripheral nuclei (blue) in Phase II, due to net plus-end droplet transport. When Kinesin-1 is impaired (Khc17 GLC), Klar is uniformly distributed in the peripheral cytoplasm below the nuclei. When either of the Kinesin-1 subunits is entirely absent (Khc27 or Klc8ex94 GLC), Klar is present not only throughout the peripheral cytoplasm, but also apical to nuclei and deep within the central yolk (where microtubules are not present). This severe mislocalization suggests that droplets are no longer attached to microtubules and diffuse throughout the embryo.
(E) Droplet association of Klar does not require Kinesin-1. When embryos are centrifuged, the low-density lipid droplets accumulate in a distinct top layer, just above the nuclei (blue). Klar (green) is highly enriched in the droplet layer of both wild-type and Khc27 GLC embryos.
