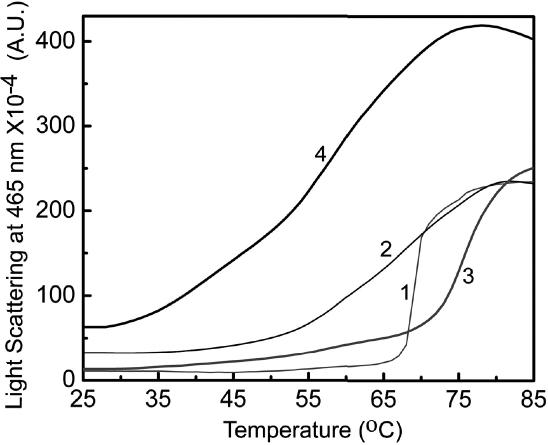
Figure 7.
Thermal stability of wild type and G98R αA-crystallin upon Cu2+-binding. Aggregation of 0.2 mg/ml of αA-crystallin (curve 1) and G98R αA-crystallin (curve 2) in buffer B and of 30 μM Cu2+-treated samples of αA-crystallin (curve 3) and G98R αA-crystallin (curve 4) is shown. The aggregation was monitored by light scattering at 465 nm as a function of temperature. G98R mutation in αA-crystallin leads to decreased thermal stability upon Cu2+-binding.