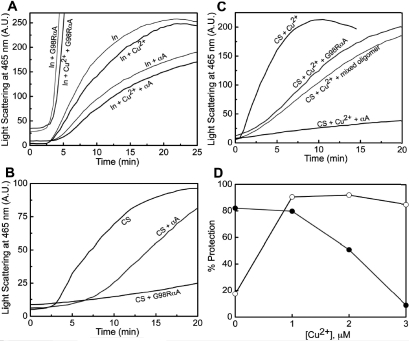
Figure 8.
Chaperone-like activity of αA-crystallin and G98R αA-crystallin with and without Cu2+ using insulin and citrate synthase as target proteins. The difference in the chaperone-like activity of the mutant protein with respect to the wild type protein toward DTT-induced aggregation of insulin at 37 °C and heat-induced aggregation of CS at 43 °C was assayed in the absence and the presence of Cu2+. A: Aggregation of 0.2 mg/ml insulin (In) in 10 mM phosphate buffer (pH 7.4) containing 100 mM NaCl was monitored by light scattering at 465 nm (expressed in arbitrary units [AU]) in the absence or in the presence of 0.1 mg/ml αA-crystallin and G98R αA-crystallin. A similar experiment was performed in the presence of 15 μM Cu2+. B: Aggregation of 25 μg/ml citrate synthase (CS) was monitored by light scattering at 465 nm in the absence and in the presence of 20 μg/ml of either αA-crystallin or G98R αA-crystallin. C: The effect of αA-crystallin, G98R αA-crystallin, and their mixed oligomer on the aggregation of CS in the presence of 3 μM Cu2+ was measured. D: Percentage protection of CS aggregation in the presence of 1 μM αA-crystallin (○) and G98RαA-crystallin (●) as a function of Cu2+ concentration indicate that the intrinsic chaperone ability of αA-crystallin is increased and that of G98R αA-crystallin is decreased. The experiments were performed three times, and the trends were reproducible. Representative data are shown.