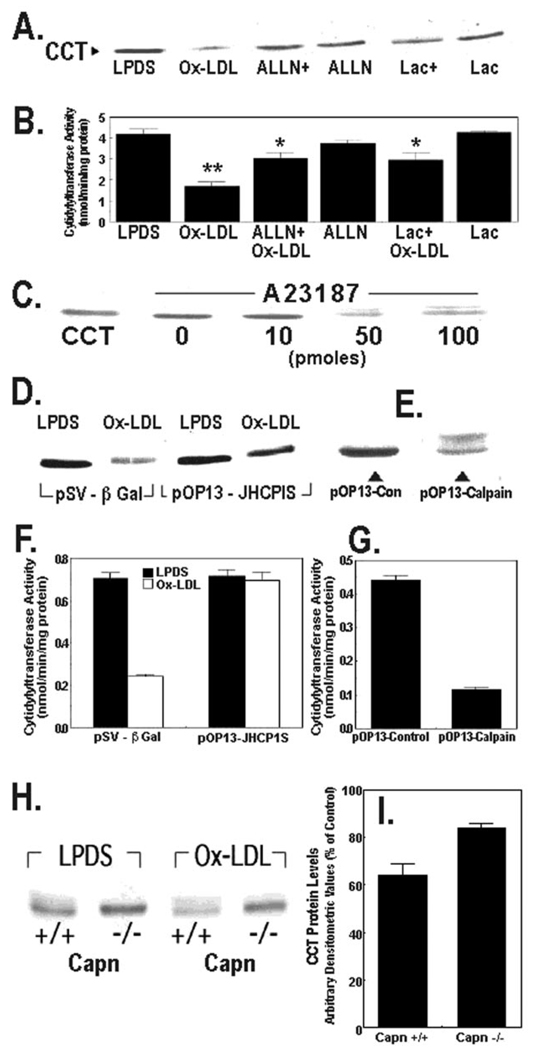
FIG. 4. Degradation of CCTα by oxidized LDL is mediated by calpain.
A and B, MLE cells cultured in LPDS with or without oxidized LDL Ox-LDL (100 µg/ml) were pretreated with ALLN (40 µg/ml) or lactacystin (Lac) (5 µm) for 1 h alone or prior to exposure to Ox-LDL for 48 h. The levels of CCTα protein (A) or activity (B) were then assayed. ALLN+, ALLN plus Ox-LDL; Lac+, lactacystin plus Ox-LDL. **, p < 0.01 versus all groups; *, p < 0.05 versus LPDS, Ox-LDL, and Lac groups by ANOVA; n = 3 separate studies. C, cells were cultured in LPDS alone or with various amounts of A23187 for 18 h. Cells were then harvested for CCTα immunoblotting. The leftmost lane contains CCTα standard (2 µg). D, cells were transfected with a control plasmid, pSV-β-galactosidase (5 µg), or the calpastatin inhibitory domain fragment, pOP13-JHCPIS (5 µg), and allowed to recover for 24 h before incubation with or without Ox-LDL (100 µg/ml) for 48 h. Cells were then harvested for CCTα immunoblotting. E, cells were transfected with a defective M-calpain control plasmid (5 µg) or a plasmid encoding M-calpain, pOP13-calpain (5 µg). Cells were processed for CCTα immunoblotting. F and G, CCT activity was determined in the cellular lysates from studies shown in panels D and E. H and I, calpain-deficient cells (Capn −/−) or wild-type controls (Capn +/+) were cultured for 48 h in LPDS with or without Ox-LDL (100 µg/ml). Cells were harvested for CCTα immunoblotting, and densitometric analysis of immunoblots was performed on the 42-kDa enzyme. Panel I represents the percent of CCTα protein detected in each cell type after Ox-LDL treatment relative to LPDS controls. All lanes in the individual panels were loaded with equal amounts of total cellular protein.