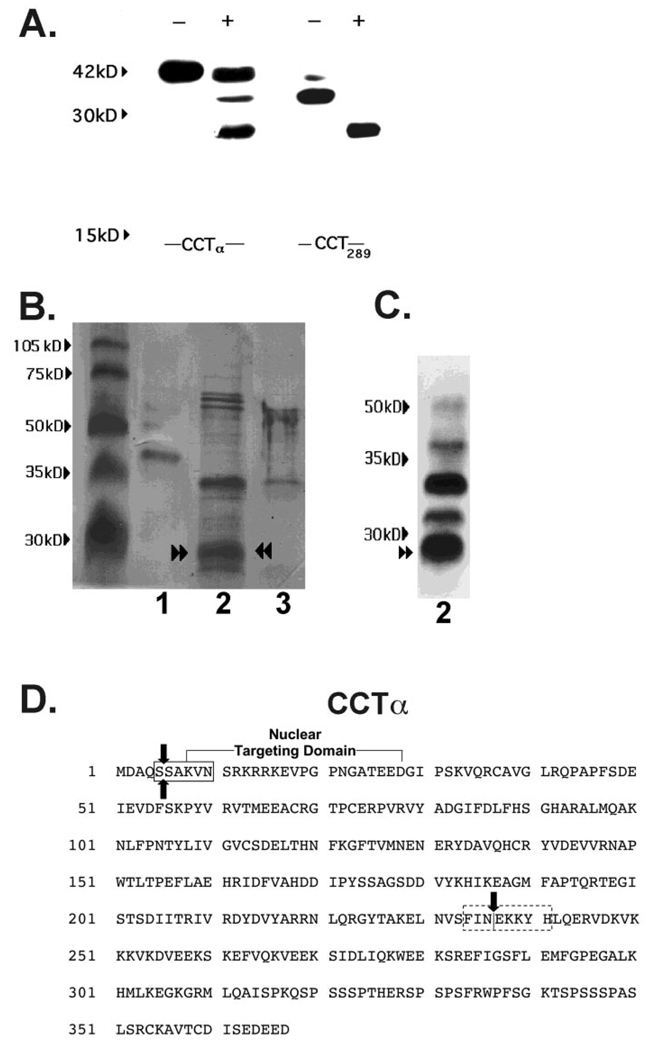
FIG. 6. In vitro digestion of CCTα by purified M-calpain.
A, MLE cells were transfected with His-tagged full-length pCMV5-CCTα (CCTα) and the internal deletion mutant, His-tagged pCMV5-CCT289 (CCT289), which lacks the membrane-binding domain. After a 24-h recovery period, lysates were harvested. Lysates were purified on a His-tagged column and treated without (−) or with (+) M-calpain (0.7 µg) for 30 min prior to SDS-PAGE and immunoblotting for CCTα. Data are representative of four separate studies. B, Coomassie Blue staining of CCTα cleavage products after calpain treatment. Recombinant purified CCTα alone (2 µg (lane 1), purified CCTα (25 µg) in combination with M-calpain (0.7 µg) (lane 2), or M-calpain alone (0.7 µg) (lane 3) were reacted in vitro for 30 min and the reaction products run on SDS-PAGE followed by transfer to Problot membranes for Coomassie Blue staining. C, immunoblotting for CCTα cleavage products from proteolysis reactions described in B, lane 2, was performed. Paired arrowsheads represent an ~26-kDa CCTα degradation product that was submitted for NH2-terminal sequencing. D, the primary sequence of CCTα showing the amino-terminal cleavage site outside of the nuclear targeting domain (boxed region) identified by NH2-terminal sequencing of the 26-kDa calpain fragment (paired arrows). The dotted box indicates the putative carboxyl-terminal calpain cleavage region suggested by MALDI-MS. The single vertical arrow indicates the catalytic membrane-binding domain boundary.