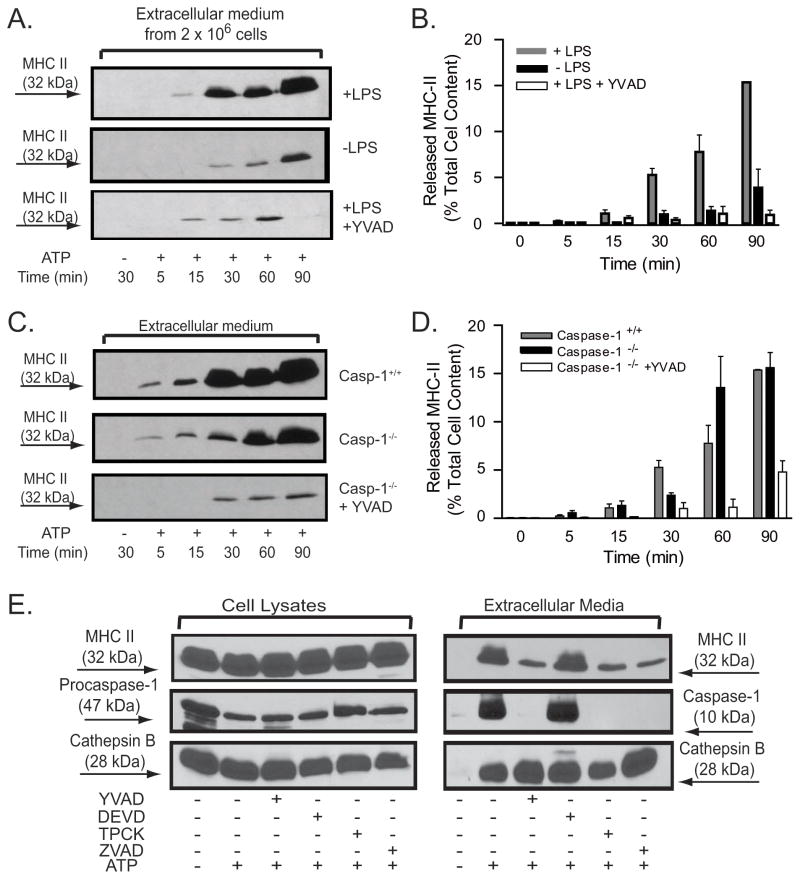
Figure 2. P2X7R-induced release of MHC-II is potentiated by LPS priming and suppressed by peptide inhibitors of proteases but is independent of caspase-1.
A, B. BMDM from wildtype C57BL/6 mice were pretreated with IFN-γ (2 ng/ml) for 16–18 h before priming with or without LPS (1 μg/ml) for 4 hr. The primed BMDM were then stimulated without or with 5 mM ATP for the indicated times in the absence or presence of 50 YVAD-cmk. The released MHC-II at each time point was quantified as the percentage of total cellular MHC-II content using the protocol in Figs. 1C and D. The data points in panel B represent the means±SE from 8 experiments that tested the effects of priming and 6 experiments that tested the effects of the YVAD-cmk inhibitor; the western blots in panel A are from representative experiments. C, D. BMDM from wildtype C57BL/6 mice (WT) or caspase−/−mice were pretreated with IFN-γ (2 ng/ml) for 16–18 h before priming with LPS (1 μg/ml) for 4 hr. The primed BMDM were then stimulated without or with 5 mM ATP for the indicated times in the absence or presence of 50 μM YVAD-cmk. The released MHC-II at each time point was quantified as the percentage of total cellular MHC-II content using the protocol in Fig. 1C and D The data points in panel D represent the means±SE from 8 experiments with WT BMDM, 5 experiments with caspase-1−/− BMDM, and 4 experiments with caspase-1−/− BMDM treated with YVAD-cmk; the western blots in panel C are from representative experiments. E.. IFN–γ and LPS-primed BMDM from wildtype C57BL/6 mice were transferred to BSS and incubated with or without 50 μM YVAD-cmk, 50 μM DEVD-cho, 50 μM ZVAD-fmk, or 100 μM TPCK for 30 min prior to stimulation with 5 mM ATP for an additional 30 min. The extracellular media and cell lysates were separately collected and processed for western blot analysis of MHC-II, caspase-1, and cathepsin B. The data are representative of results from 3 separate experiments.