Abstract
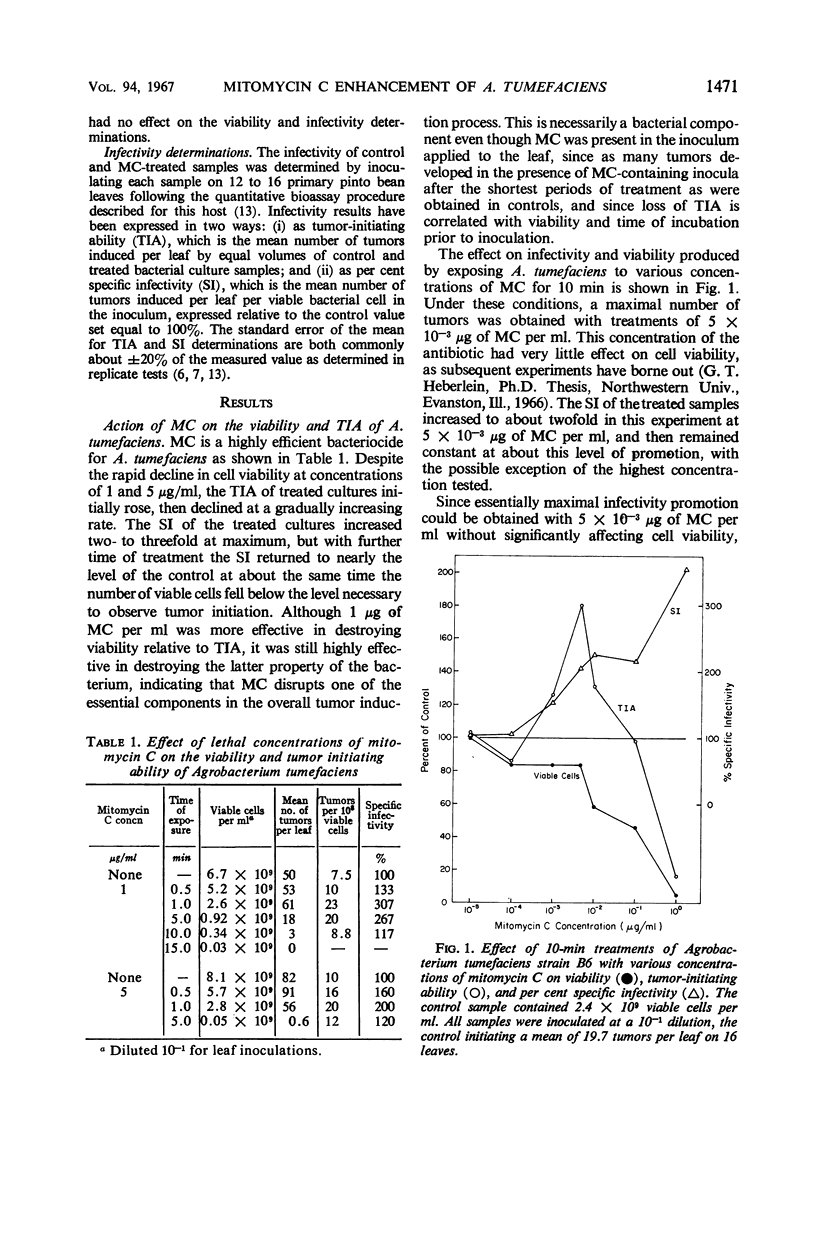
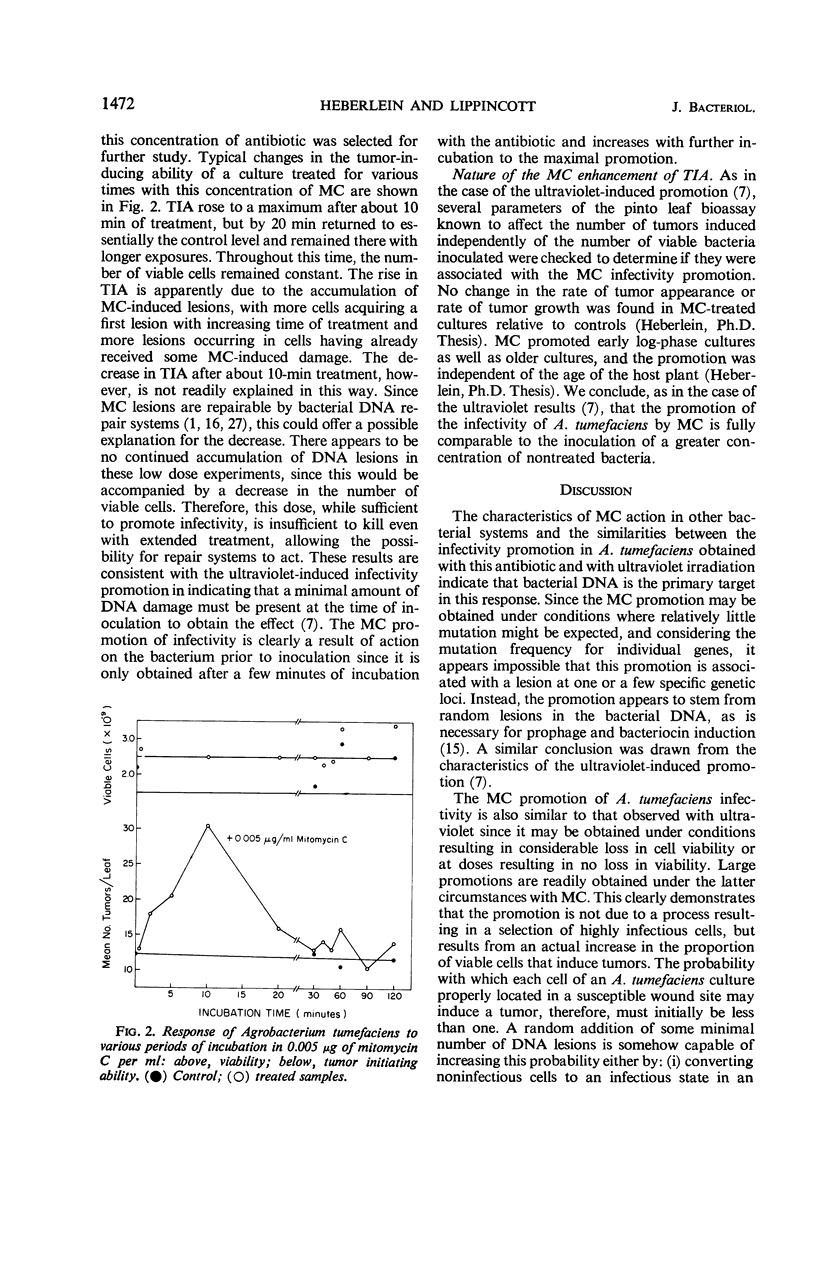
The ability of Agrobacterium tumefaciens to induce pinto leaf tumors may be enhanced two- to threefold after treatment with mitomycin C. The enhancement may be obtained with either lethal or nonlethal concentrations. With 10-min treatments, an optimal response was obtained with 0.005 μg of mitomycin C per ml in the absence of any change in the number of viable cells. Both the tumor induction process and the tumors induced by treated cultures appear qualitatively the same as controls. To account for these results, the antibiotic must increase the proportion of viable cells that will subsequently initiate tumors. One, or at most a few, random lesions in the bacterial chromosome seem to be the necessary requirement for this promotion. At mitomycin concentrations of 1 and 5 μg/ml, the ability of A. tumefaciens to initiate tumors is rapidly lost, indicating that a fairly intact bacterial chromosome is one of the essentials for the tumor induction process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- Coles N. W., Gross R. The effect of mitomycin C on the induced synthesis of penicillinase in Staphylococcus aureus. Biochem Biophys Res Commun. 1965 Jul 26;20(3):366–371. doi: 10.1016/0006-291x(65)90374-8. [DOI] [PubMed] [Google Scholar]
- De Ley J., Bernaerts M., Rassel A., Guilmot J. Approach to an improved taxonomy of the genus Agrobacterium. J Gen Microbiol. 1966 Apr;43(1):7–17. doi: 10.1099/00221287-43-1-7. [DOI] [PubMed] [Google Scholar]
- Dudnik Y. V. Induction of lysogenic culture of Micrococcus lysodeikticus by antibiotics selectively affecting DNA synthesis. Fed Proc Transl Suppl. 1965 Nov-Dec;24(6):1049–1051. [PubMed] [Google Scholar]
- GRULA M. M., GRULA E. A. Reversal of mitomycin c-induced growth and division inhibition in a species of Erwinia. Nature. 1962 Sep 15;195:1126–1127. doi: 10.1038/1951126a0. [DOI] [PubMed] [Google Scholar]
- HEBERLEIN G. T., LIPPINCOTT J. A. PHOTOREVERSIBLE ULTRAVIOLET ENHANCEMENT OF INFECTIVITY IN AGROBACTERIUM TUMEFACIENS. J Bacteriol. 1965 Jun;89:1511–1514. doi: 10.1128/jb.89.6.1511-1514.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein G. T., Lippincott J. A. Ultraviolet-induced changes in the infectivity of Agrobacterium tumefaciens. J Bacteriol. 1967 Apr;93(4):1246–1253. doi: 10.1128/jb.93.4.1246-1253.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. MITOMYCINS AND PORFIROMYCIN: CHEMICAL MECHANISM OF ACTIVATION AND CROSS-LINKING OF DNA. Science. 1964 Jul 3;145(3627):55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- KILGORE W. W., GREENBERG J. Filament formation and resistane to 1-methyl-3-nitro-1-nitrosoguanidine and other radiomimetic compounds in Escherichia coli. J Bacteriol. 1961 Feb;81:258–266. doi: 10.1128/jb.81.2.258-266.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE M. Effect of mitomycin C on interactions between temperate phages and bacteria. Virology. 1961 Apr;13:493–499. doi: 10.1016/0042-6822(61)90280-x. [DOI] [PubMed] [Google Scholar]
- LIPPINCOTT J. A., LIPPINCOTT B. B. AGROBACTERIUM TUMEFACIENS: THERMAL INACTIVATION OF TUMOR-INDUCING ABILITY. Science. 1965 Mar 26;147(3665):1578–1579. doi: 10.1126/science.147.3665.1578. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Heberlein G. T. The quantitative determination of the infectivity of Agrobacterium tumefaciens. Am J Bot. 1965 Sep;52(8):856–863. [PubMed] [Google Scholar]
- MARJAI E., IVANOVICS G. THE EFFECT OF DIFFERENT ANTICANCER AGENTS ON INDUCIBLE SYSTEMS OF BACILLUS MEGATERIUM. Acta Microbiol Acad Sci Hung. 1964;11:193–198. [PubMed] [Google Scholar]
- MATSUMOTO I., LARK K. G. ALTERED DNA ISOLATED FROM CELLS TREATED WITH MITOMYCIN C. Exp Cell Res. 1963 Oct;32:192–196. doi: 10.1016/0014-4827(63)90089-2. [DOI] [PubMed] [Google Scholar]
- Mahler I. Effect of mitomycin C on five excision-repair mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1966 Oct 5;25(1):73–79. doi: 10.1016/0006-291x(66)90642-5. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- OTSUJI N. The effect of glucose on the induction of lambda phage formation by mitomycin C. Biken J. 1961 Dec;4:235–241. [PubMed] [Google Scholar]
- Pricer W. E., Jr, Weissbach A. The effect of lysogenic induction with mitomycin C on the DNA and DNA polymerase of Escherichia coli K12-mu. Biochem Biophys Res Commun. 1964;14:91–95. doi: 10.1016/0006-291x(63)90217-1. [DOI] [PubMed] [Google Scholar]
- REICH E., SHATKIN A. J., TATUM E. L. Bacteriocidal action of mitomycin C. Biochim Biophys Acta. 1961 Oct 14;53:132–149. doi: 10.1016/0006-3002(61)90800-9. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI M., TAKAGI Y. Effect of mitomycin C on the synthesis of bacterial and viral deoxyribonucleic acid. Biochim Biophys Acta. 1960 Jul 15;41:434–443. doi: 10.1016/0006-3002(60)90040-8. [DOI] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- SZYBALSKI W., IYER V. N. CROSSLINKING OF DNA BY ENZYMATICALLY OR CHEMICALLY ACTIVATED MITOMYCINS AND PORFIROMYCINS, BIFUNCTIONALLY "ALKYLATING" ANTIBIOTICS. Fed Proc. 1964 Sep-Oct;23:946–957. [PubMed] [Google Scholar]
- SZYBALSKI W. Special microbiological systems. II. Observations on chemical mutagenesis in microorganisms. Ann N Y Acad Sci. 1958 Dec 5;76(3):475–489. doi: 10.1111/j.1749-6632.1958.tb57106.x. [DOI] [PubMed] [Google Scholar]
- Terawaki A., Greenberg J. Post-treatment breakage of mitomycin C induced cross-links in deoxyribonucleic acid of Escherichia coli. Biochim Biophys Acta. 1966 Jun 22;119(3):540–546. doi: 10.1016/0005-2787(66)90130-4. [DOI] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION AND PROPERTIES OF A TEMPERATURE BACTERIOPHAGE FROM BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Jan;89:175–184. doi: 10.1128/jb.89.1.175-184.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


