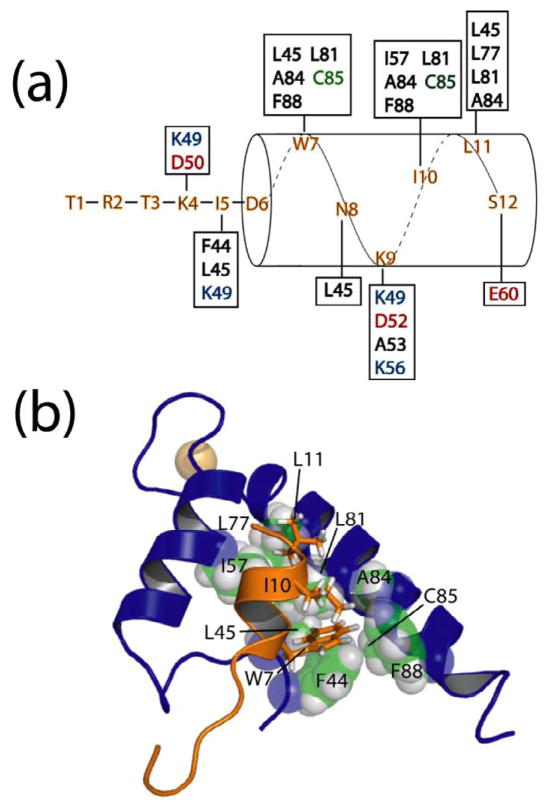
Figure 3.
Hydrophobic residues of TRTK12 interact with a hydrophobic binding pocket on Ca2+-S100A1. (a) Residues that give intermolecular NOE correlations between the TRTK12 peptide and residues on S100A1 (in boxes) are illustrated. S100A1 residues are colored black for hydrophobic, green for polar, red for negatively charged, and blue for positively charged residues. (b) Residues of TRTK12 including W7, I10, and L11 are packed nearby hydrophobic residues from the hinge region (F44, L45), helix 3 (I57), and helix 4 (L77, L81, A84, C85, and F88) of S100A1. In particular, W7 fits into a well-formed hydrophobic pocket consisting of F44, L45, L81, A84, C85, and F88 of Ca2+-S100A1.