Abstract
Repeated high-dose methamphetamine administrations can cause persistent dopaminergic deficits. As individuals abusing methamphetamine are often exposed to recurrent high-dose administration, the impact of its repeated exposure merits investigation. Accordingly, rats were pretreated with repeated high-dose injections of methamphetamine, and subsequently “challenged” with the same neurotoxic regimen 7 or 30 days later. Results revealed that the initial methamphetamine treatment caused persistent deficits in striatal dopamine levels, dopamine transporter function, and vesicular monoamine transporter-2 function. The subsequent methamphetamine challenge treatment was without further persistent effects on these parameters, as assessed 7 days after the challenge, regardless of the interval (7 or 30 days) between the initial and challenge drug exposures. Similarly, a methamphetamine challenge treatment administered 7 days after the initial drug treatment was without further acute effect on dopamine transporter or VMAT-2 function, as assessed 1 hour later. Thus, this study describes a model of resistance, possibly explained by: 1) the existence of dopaminergic neurons that are a priori refractory to deficits caused by methamphetamine; 2) the existence of dopaminergic neurons made persistently resistant consequent to a neurotoxic methamphetamine exposure; and/or 3) altered activation of post-synaptic basal ganglia systems necessary for the elaboration of methamphetamine-induced dopamine neurotoxicity.
Keywords: methamphetamine, neurotoxicity, dopamine, tolerance, resistance
1. Introduction
Previous studies have demonstrated that repeated high-dose administrations of methamphetamine cause persistent dopaminergic neuronal deficits in rodents, non-human primates and humans. For example, high-dose methamphetamine administration is associated with long-term striatal reduction in: 1) dopamine levels (Kogan et al., 1976; Ricaurte et al., 1980; Wagner et al., 1980; Woolverton et al., 1989); 2) dopamine transporter (Wagner et al., 1980; Guilarte et al., 2003) and vesicular monoamine transporter-2 (VMAT-2) levels (Guilarte et al., 2003); and 3) tyrosine hydroxylase activity (Kogan et al., 1976; Hotchkiss et al., 1979). In addition to these persistent deficits, repeated methamphetamine administrations rapidly (within 1 h) reduce striatal dopamine transporter activity, as measured in synaptosomes obtained from treated rats (Fleckenstein et al., 1997; Kokoshka et al., 1998). Methamphetamine treatment also decreases striatal VMAT-2 activity acutely, as measured ex vivo in a cytoplasmic (non-membrane associated) vesicular subcellular fraction prepared from treated rats (Brown et al., 2000); an outcome presumptively linked with a redistribution of VMAT-2 protein within nerve terminals (Riddle et al., 2002; for review, see also Fleckenstein et al., 2007 and references contained therein).
Many laboratories have investigated the impact of repeated methamphetamine injections in rodent models. However, relatively few studies have investigated the impact of repeated “binge-like” methamphetamine treatments. This is an important clinical issue since many individuals who abuse methamphetamine are subjected to multiple “binge” episodes, each of which includes exposure to several high-dose administrations of drug. Noteworthy, one study by Thomas and Kuhn (2005) has investigated this issue. In particular, mice were treated with a high-dose, “neurotoxic” methamphetamine regimen and "challenged" 7 days later with a second “binge-like” exposure. Results revealed that the first methamphetamine treatment decreased striatal dopamine tissue content, and dopamine levels were not further depleted by the second methamphetamine exposure. In addition, the second methamphetamine treatment did not provoke a subsequent microglial response when the mice were pretreated with the first “neurotoxic” methamphetamine regimen, regardless of the interval between methamphetamine exposures (7 vs. 30 days). Importantly, dopamine levels and microglial assessments in those studies were assessed 2 days after the second methamphetamine exposure, and these investigators suggested that the microglial activation “precedes and contributes to methamphetamine-induced nerve ending damage.”
Given the importance of understanding the impact of repeated methamphetamine exposures, the purpose of the present study, like that of Thomas and Kuhn (2005), was to assess the effect of pretreatment with a “binge-like” methamphetamine regimen on subsequent responses of striatal dopaminergic systems to a second “challenge” with multiple high-dose administrations of the stimulant. In contrast to this previous report, the current study focused on the response of the dopamine transporter and VMAT-2 both acutely (1 hour) after the second treatment and 7 days later (i.e., a time point typically used to assess the expression of toxicity because dopamine terminals have presumably been severely damaged or lost by this time). Results revealed that the first treatment regimen caused persistent deficits in dopamine levels, as well as in dopamine transporter and VMAT-2 function. However, a subsequent methamphetamine challenge treatment was without further persistent effects on these parameters, regardless of the interval between exposures. Similarly, a subsequent methamphetamine challenge treatment administered 7 days after the initial drug treatment was without further acute effects on dopamine transporter or VMAT-2 function, as assessed 1 h later. This model of resistance is unique relative to at least one other model wherein apparent tolerance to the effects of a neurotoxic methamphetamine regimen is transient (e.g., Danceau et al., 2007), and can be explained by either: 1) the existence of a population of dopaminergic neurons that is a priori resistant to the persistent deficits caused by methamphetamine; 2) the existence of a population of dopaminergic neurons made resistant as a consequence of exposure to a neurotoxic regimen of methamphetamine; and/or 3) altered activation of post-synaptic basal ganglia systems necessary for the elaboration of methamphetamine-induced monoamine neurotoxicity. Identification of this phenomenon may facilitate understanding the neurobiological mechanisms underlying persistently altered behaviors observed as a consequence of methamphetamine-induced monoamine neurotoxicity.
2. Materials and Methods
2.1. Animals
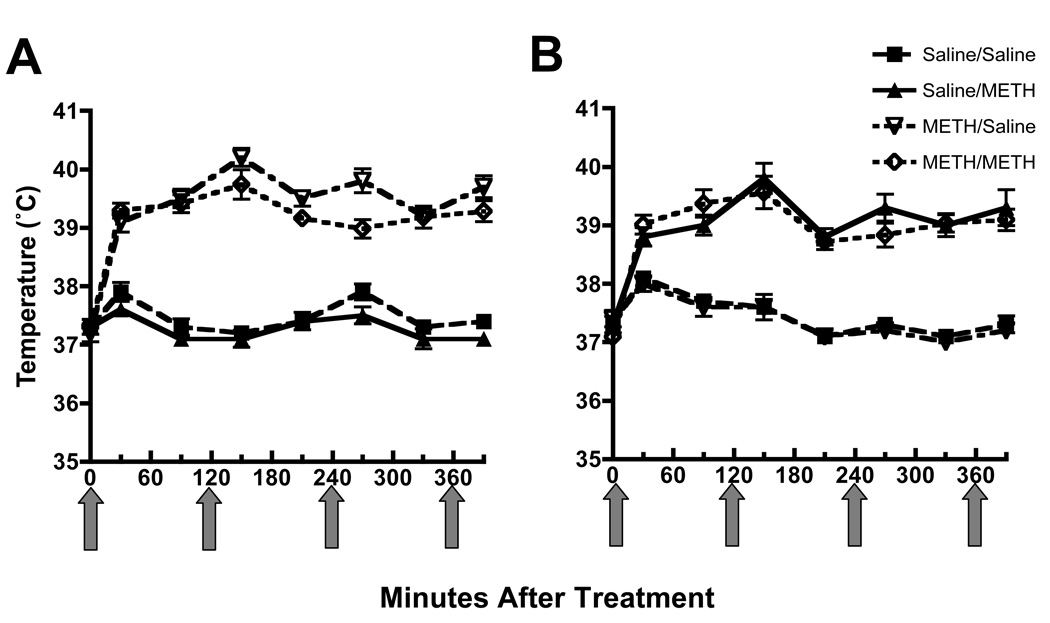
Male Sprague-Dawley rats (averaging 250 – 350 g; Charles River; Raleigh NC) were maintained under conditions of controlled lighting and temperature. Food and water were available ad libitum. Rats received 4 injections (s.c., 7.5 mg/kg/injection, 2-hour intervals) of methamphetamine or saline vehicle (s.c., 1 ml/kg/injection, 2-hour intervals). Rectal temperatures were recorded using a digital thermometer (Physiotemp Instruments, Clifton, NJ). Zero-time values in Fig. 2 were obtained 30 min prior to the first injection, and rectal temperatures were assessed 30 min prior to and 30 min after each injection. Seven or 30 days later, rats again received this saline or methamphetamine regimen. Rats were sacrificed by decapitation 1 hour or 7 days after the second methamphetamine exposure to assess the acute and persistent effects of the stimulant, respectively. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Utah.
Figure 2.
Methamphetamine treatment increased core body temperature (A) and this effect was not was not altered in rats that received the subsequent methamphetamine “challenge” treatment (B). Rats were treated as described for Figure 1A. Zero-time values were obtained 30 min prior to the first injection, and rectal temperatures were assessed 30 min prior to and min after each injection. Arrows represent the time of each saline or methamphetamine injection. Values represent means and vertical lines 1 S.E.M. of determinations in 9 – 11 rats.
2.2. Drugs and Chemicals
(±)-Methamphetamine hydrochloride was supplied by Research Triangle Institute (Research Triangle Park, NC). 3,4-[Ring-2,5,6-3H]Dihydroxyphenylethlamine (dopamine; 39.3 and 54.7 Ci/mmol) was purchased from Perkin Elmer (Boston, MA). Doses were calculated as the respective free base.
2.3 Preparation of Synaptosomes
The striata from treated rats were quickly dissected and homogenized (15 mg original wet weight/ml) in ice-cold 0.32 M sucrose (pH 7.4) containing 3.8 mM NaH2PO4 and 12.7 mM Na2HPO4, then centrifuged (800 × g, 12 min, 4 °C). The resulting supernatant was centrifuged (22,000 × g, 15 min, 4 °C) to obtain a pellet containing synaptosomes (P2).
2.4. Preparation of Synaptic Vesicles
The P2 synaptosomal pellet was prepared as described above, and lysed using deionized water. HEPES and potassium tartrate were then added to final concentrations of 25 and 100 mM, respectively (pH 7.5). To remove lysed synaptosomal membranes, samples were centrifuged (20,000 × g, 20 minutes, 4 °C). MgSO4 (final concentration 1 mM) was added to the supernatant which was subsequently centrifuged (100,000 × g, 45 minutes, 4 °C). The resulting pellet (i.e., the non-membrane-associated or “cytoplasmic” fraction) was resuspended in wash buffer (assay buffer containing 1 mM MgSO4 substituted for the ATP-Mg2+; pH 7.5) at a concentration of 50 mg/ml (original tissue wet weight of striatum).
2.5. Plasmalemmal [3H]Dopamine Uptake
Plasmalemmal [3H]dopamine uptake was assessed by incubating striatal synaptosomes (10 min, 37 °C) in assay buffer (in mM: 126 NaCl, 4.8 KCl, 1.3 CaCl2, 16 sodium phosphate, 1.4 MgSO4, 11 glucose and 1 ascorbic acid; pH 7.4) and 1 µM pargyline. Nonspecific values were determined in the presence of 50 µM cocaine for the dopamine transporter assays. The assays were initiated by the addition of [3H]dopamine (0.5 nM final concentration). Following incubation (3 minutes), samples were placed on ice to stop the reaction and filtered through GF/B filters (Whatman, Clifton NJ) soaked previously in 0.05% polyethylenimine. Filters were rapidly washed three times with 3 ml of ice cold 0.32 M sucrose using a filtering manifold (Brandel, Gaithersburg MD). Radioactivity trapped in filters was counted using a liquid scintillation counter. All proteins were determined using the Lowry Protein Assay (Lowry et al., 1951).
2.6. Vesicular [3H]Dopamine Uptake
Vesicular [3H]dopamine uptake was evaluated by incubating striatal synaptic vesicle preparations (3 minutes, 30 °C) in assay buffer (final concentration 25 mM HEPES, 100 mM potassium tartrate, 1.7 mM ascorbic acid, 0.05 mM EGTA, 0.1 mM EDTA and 1.8 mM ATP-Mg2+; pH 7.5) in the presence of [3H]dopamine (30 nM final concentration). Samples were rapidly filtered using a filtering manifold (Brandel, Inc.) through GF/F filters (VWR, West Chester PA) previously soaked in 0.5% polyethylenimine and washed 3 times with cold wash buffer. Using a liquid scintillation counter, the radioactivity trapped in filters was counted. Nonspecific values were determined by measuring vesicular [3H]dopamine uptake at 4 °C in the absence of ATP. All proteins were determined using the Bradford Protein Assay (Bradford, 1976).
2.7. Dopamine Content
Striatal tissues were sonicated in 1 ml of ice-cold tissue buffer (0.05 M sodium phosphate and 0.03 M citric acid with 15 % methanol (v/v); pH 2.5) and centrifuged (22,000 × g, 15 min, 4 °C). The pellet was retained for protein determination using the Lowry Protein Assay (Lowry et al., 1951) and the supernatant was centrifuged 4 more times (22,000 × g, 10 min, 4 °C). An aliquot of the resulting supernatant was then loaded directly onto a high performance liquid chromatography system coupled to an electrochemical detector (+0.73 V) for separation and quantification of dopamine levels (mobile phase consisted of 0.05 M sodium phosphate, 0.03 M citric acid, 0.16 mM EDTA, 0.035 % sodium octylsulfate, 15 % methanol, pH 2.86; Whatman 25-cm C-18 column).
2.8. Data Analysis
Statistical analysis was conducted using an analysis of variance followed by a Fischer’s Protected Least Significant Difference post hoc comparison. Differences were considered significant if the probability of error was less than 5%.
3. Results
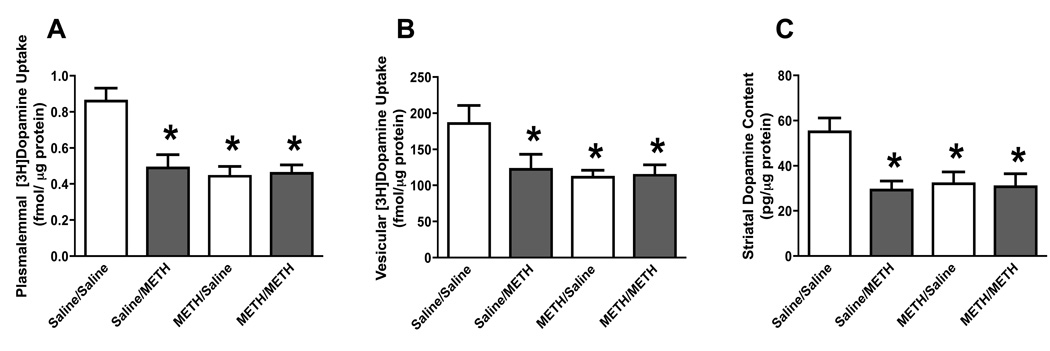
Experiments were designed to determine the effects of methamphetamine after preexposure to a “neurotoxic” regimen of methamphetamine demonstrated to cause long-term dopaminergic deficits. In particular, rats received 4 injections (s.c.; 2-hour intervals) of methamphetamine (7.5 mg/kg/injection) or saline (1 ml/kg/injection). Seven days later, animals were “challenged” with identical treatments of either saline or methamphetamine. Rats were then sacrificed 7 days after the challenge drug administration (i.e., 14 days after the first saline or methamphetamine exposures). Results reveal that the initial “neurotoxic” regimen per se caused persistent deficits in dopamine transporter function (Fig. 1A), VMAT-2 function (Fig. 1B), and dopamine levels (Fig. 1C), as assessed 14 days after the initial treatment. The effects of the initial methamphetamine exposure were not enhanced in rats that received the subsequent methamphetamine “challenge” treatment. It is important to note that in all experiments (including those described below), rats that received methamphetamine treatment were maintained in a warm environment (e.g., greater than 24 °C) during their drug exposure and thus had increased core body temperatures when compared to those control rats receiving saline. The methamphetamine-induced increases in temperature were similar after the challenge regimen compared to the neurotoxic regimen. Representative temperature data corresponding to Figure 1A are presented in Figure 2.
Figure 1.
A challenge methamphetamine treatment was without effect on striatal plasmalemmal dopamine uptake (A), vesicular dopamine uptake (B), and dopamine content (C) in rats pretreated with methamphetamine. Rats designated as “Sal/Sal” were pretreated with 4 injections (s.c., 2-hour intervals) of saline (1 ml/kg/injection). Seven days later, rats were again treated with 4 injections (s.c., 2-hour intervals) of saline (1 ml/kg/injection). Rats designated as “Sal/METH” were pretreated with 4 injections (s.c., 2-hour intervals) of saline (1 ml/kg/injection). Seven days later, rats were treated with 4 injections (s.c., 2-hour intervals) of methamphetamine (7.5 mg/kg/injection). Rats designated as “METH/Sal” were pretreated with 4 injections (s.c., 2-hour intervals) of methamphetamine (7.5 mg/kg/injection). Seven days later, rats were treated with 4 injections (s.c., 2-hour intervals) of saline (1 ml/kg/injection). Rats designated as “METH/METH” were pretreated with 4 injections (s.c., 2-hour intervals) of methamphetamine (7.5 mg/kg/injection). Seven days later, rats were treated with 4 injections (s.c., 2-hour intervals) of methamphetamine (7.5 mg/kg/injection). Rats were sacrificed 7 days after the final saline or methamphetamine treatment. Columns represent means and vertical lines 1 S.E.M. of determinations in 5 – 11 rats. *Values significantly different from saline-treated controls (P ≤ 0.05).
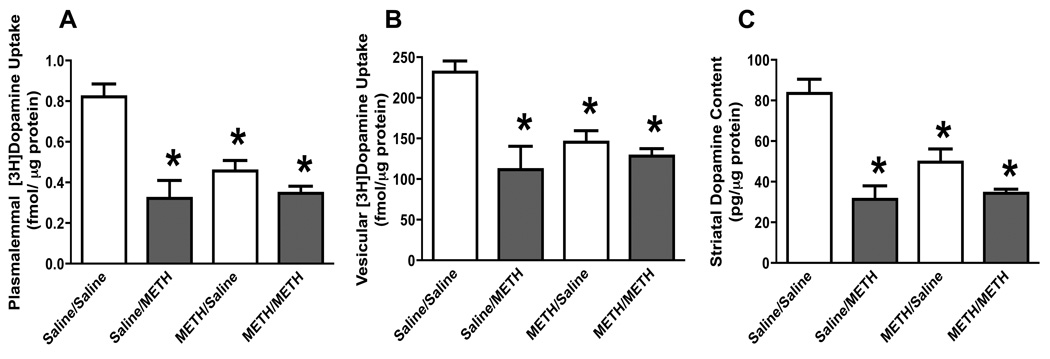
To determine if the effects of a pretreatment with multiple methamphetamine injections were long-standing, experiments were conducted using the same dosing regimens described for Figure 1, but wherein the time between the methamphetamine pretreatment and challenge administration was increased from 7 to 30 days. Rats were then sacrificed 7 days after the subsequent saline or “challenge” methamphetamine administration (i.e., 37 days after the first saline or methamphetamine treatments). Results revealed that the “neurotoxic” methamphetamine regimen per se caused persistent deficits in dopamine transporter function (Fig. 3A), VMAT-2 function (Fig. 3B), and dopamine levels (Fig. 3C) as assessed 37 days after the initial methamphetamine treatment. Similar to data presented in Figure 1, these effects of methamphetamine were not enhanced in rats that received the subsequent methamphetamine “challenge” treatment 30 days after the initial exposure.
Figure 3.
A challenge methamphetamine treatment was without effect on striatal plasmalemmal dopamine uptake (A), vesicular dopamine uptake (B), and dopamine content (C) in rats pretreated with methamphetamine. Rats were treated as described for Figure 1, except that the interval between the pretreatment and challenge administrations was 30 days. Columns represent means and vertical lines 1 S.E.M. of determinations in 6 – 17 rats. *Values significantly different from saline-treated controls (P ≤ 0.05).
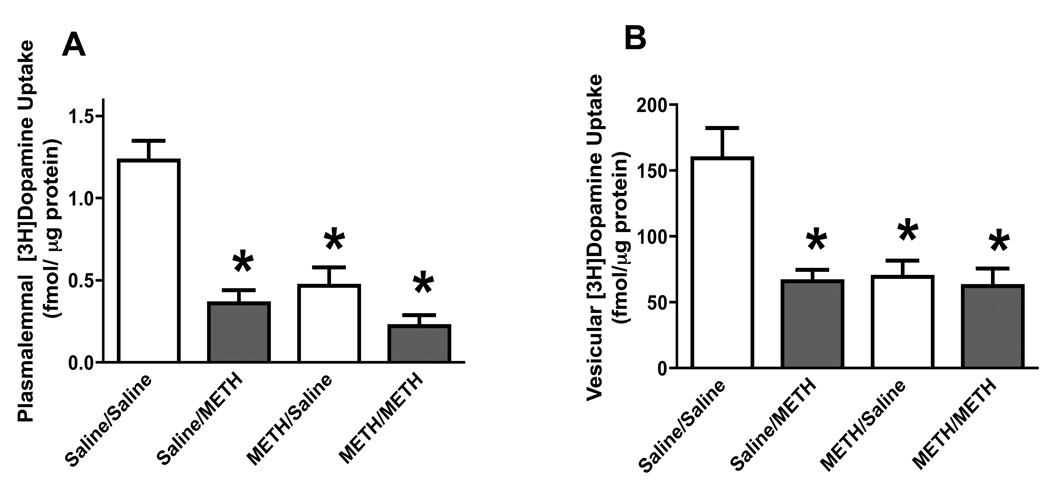
Results presented in Figure 1 and Figure 3 suggest that the persistent (7 days) response of dopamine systems to multiple high-dose injections of methamphetamine are not further enhanced by a subsequent high-dose methamphetamine challenge. To determine if the more rapid responses of this dopamine system to a “neurotoxic” methamphetamine regimen were also affected in these pretreated animals, rats were treated as described for Figure 1 – Figure 3 except that the animals were sacrificed 1 hour rather than after 7 days after the subsequent methamphetamine or saline challenge. Results once again revealed that the “neurotoxic” methamphetamine regimen caused persistent deficits in dopamine transporter (Figure 4A) and VMAT-2 function (Figure 4B); however and resembling effects presented in Figure 1 and Figure 2, the methamphetamine “challenge” treatment was without further effect on striatal plasmalemmal (Figure 4A) or vesicular (Figure 4B) dopamine uptake in methamphetamine-pretreated rats, as assessed 1 hour after the final drug treatment.
Figure 4.
A challenge methamphetamine treatment was without effect on striatal plasmalemmal dopamine uptake (A) and vesicular dopamine uptake (B) in rats pretreated with methamphetamine. Rats were treated as described for Fig. 1, but sacrificed 1 hour after the final drug or saline treatment. Columns represent means and vertical lines 1 S.E.M. of determinations in 6 – 8 rats. *Values significantly different from saline-treated controls (P ≤ 0.05).
4. Discussion
Methamphetamine abuse is a serious problem in the United States. Individuals addicted to methamphetamine not only must cope with the negative societal repercussions resulting from abuse, but both the immediate and persistent neurobiological consequences as well.
Persons dependent on methamphetamine often binge repeatedly by self-administering multiple high-dose injections of the drug. To understand the effects bingeing may have on individuals addicted to methamphetamine, many laboratories have attempted to model this pattern by investigating the impact of repeated methamphetamine injections in rodents, and have reported persistent deficits in striatal dopamine systems including decreases in levels of dopamine and activities of dopamine transporter, VMAT-2 and tyrosine hydroxylase (see Introduction). This is of significance, as several reports have demonstrated that the exposure of animals to a neurotoxic methamphetamine regimen results in long-term impairment of memory and learning tasks (Chapman et al., 2001; Friedman et al., 1998; Schroder et al., 2003; Daberkow et al., 2005; Belcher et al., 2008) and alterations in basal ganglia function (Chapman et al., 2001; Johnson-Davis et al., 2002). The potential clinical relevance of these animal findings has been shown in humans; thus, methamphetamine abusers demonstrate general impairment across several neurocognitive domains, including deficits in executive function and memory (Gonzalez et al., 2007; Volkow et al., 2001; Simon et al., 2000; Kalechstein et al., 2003; Scott et al., 2007; McCann et al., 2008). At least some of the deficits observed in rodents and humans appear to correlate with impairment of dopaminergic systems (Belcher et al., 2008; Daberkow et al., 2005; Volkow et al., 2001).
Whereas the impact of single “binge-like” methamphetamine treatments are well characterized, less data are available regarding effects of repeated “binge episodes.” As discussed in the Introduction, Thomas and Kuhn (2005) conducted studies wherein mice were treated with a high-dose methamphetamine regimen and "challenged" 7 days later with a second “binge-like” exposure. These investigators reported that the first methamphetamine treatment decreased striatal dopamine tissue content, and that dopamine levels were not further depleted by the second methamphetamine exposure. In addition, these investigators suggested an association between tolerance to the neurotoxic effects of methamphetamine and attenuated microglial activation. Noteworthy, Thomas and Kuhn (2005) selected 2 days after “challenge” treatment for assessment of dopamine levels; a time at which these investigators hypothesized precedes methamphetamine-induced nerve ending damage.
In contrast to the work by Thomas and Kuhn (2005), the first novel finding of the present study is that whereas the initial exposure to the “neurotoxic” regimen caused long-term (i.e., 14 days) deficits in 3 commonly employed indices of dopaminergic neuronal integrity and function (striatal plasmalemmal dopamine uptake, vesicular dopamine uptake, and dopamine content), a second “challenge” exposure was without additional effect, as assessed 7 days after the second methamphetamine exposure. The time of assessment after the challenge (7 days) is of importance as it presumably occurs after microglial responses have subsided and at a time when dopamine nerve terminals are presumably damaged/lost. Noteworthy, the present data do not rule out the possibility that a challenge with a higher dose regimen of methamphetamine might have added to the deficits caused by the initial methamphetamine treatment, although we have observed similar resistance to further monoamine neuronal toxicity in animals pretreated and then challenged again > 30 days later with a higher dose “neurotoxic” regimen of methamphetamine (4 × 10 mg/kg; data not shown). Therefore, the data suggest that the residual dopaminergic neurons are “refractory” to a subsequent neurotoxic regimen of methamphetamine.
One potential explanation for the “refractoriness” of some dopamine neurons to subsequent “challenge” with methamphetamine is that the initial methamphetamine regimen damaged a relatively vulnerable population of dopamine neurons allowing only resistant cells to remain intact. An alternative explanation is that exposure to high doses of methamphetamine may have caused surviving neurons to change in such a way as to become resistant to further effects of the stimulant. This latter possibility may represent a defense mechanism to prevent further damage to surviving neurons in order to preserve important functions mediated by the nigrostriatal system.
A third possibility, at least with respect to the persistent dopaminergic deficits caused by methamphetamine, is that the partial monoamine loss induced by the initial neurotoxic regimen prevents the second neurotoxic regimen from activating post-synaptic basal ganglia circuitry necessary for the neurotoxicity to occur. Noteworthy, prior destruction of neurons by intrastriatal infusion of quinolinic acid prevents methamphetamine-induced dopaminergic deficits, suggesting a contribution of such post-synaptic circuitry to the development of toxicity (O’Dell et al., 1994). Further, studies by Yamamoto and co-workers (e.g., Mark et al., 2004) have demonstrated that activation of striatonigral efferent neurons by neurotoxic regimens of methamphetamine increases striatal glutamate outflow that then contributes to the persistent dopaminergic deficits caused by methamphetamine. Importantly in this regard, previous work from our laboratories (Chapman et al., 2001; Johnson-Davis et al., 2002; Daberkow et al., Neurotoxicity Research, in press) has demonstrated long-term decreases in indices of D1 receptor-containing striatonigral efferent neuronal function subsequent to methamphetamine-induced neurotoxicity. Taken together, these data suggest that activation of striatonigral circuitry critical for producing methamphetamine-induced monoamine toxicity likely is impaired in rats that already have a partial monoamine loss induced by prior exposure to a neurotoxic regimen of methamphetamine and that this lack of activation of post-synaptic circuitry or other D1 receptor-mediated effects (e.g. activation of neuronal nitric oxide synthase-containing interneurons) may underlie the refractoriness to further neurotoxicity in these animals.
Of related interest are findings by Cass and Manning (1999) of less amphetamine-induced dopamine release when amphetamine is administered 1 – 4 weeks after an initial neurotoxic methamphetamine insult. Less dopamine release could lead to less production of extraneuronal dopamine-associated reactive species and thus less microglial activation following the methamphetamine challenge; a process that has been suggested to contribute significantly to methamphetamine-induced dopaminergic deficits (Thomas et al., 2008). As dopamine contributes to methamphetamine-induced reactive species formation, less dopamine release could lead to diminished extracellular dopamine-associated oxidative stress in response to methamphetamine as well.
Several investigators have reported resistance to the persistent dopaminergic deficits caused by a “challenge” methamphetamine administration in rats pretreated with either an intermittent, low-dose or multiple escalating-dose injections of the stimulant (Schmidt et al., 1985; Stephans and Yamamoto, 1996; Johnson-Davis et al., 2003, 2004; Segal et al., 2003; Thomas and Kuhn, 2005; Danaceau et al., 2007; Graham et al., 2008). These pretreatments often included administration of relatively lower doses of methamphetamine, thus generally causing relatively little or no persistent dopamine deficits at the time point at which animals were finally challenged with multiple, high-dose injections of methamphetamine. In contrast, in the current study, rats were pretreated with a “neurotoxic” methamphetamine regimen resulting in substantial and long-term deficits prior to the subsequent “challenge” with a second “neurotoxic” regimen of the stimulant. Interestingly, a recent study demonstrated that the protection afforded by at least one escalating-dose regimen is diminished and eventually disappears if the duration between the initial insult and the challenge treatment was increased to 14 – 31 days (Danaceau et al., 2007). In contrast, the present study demonstrated that the refractoriness of striatal dopamine systems to deficits induced by exposure to a second “neurotoxic” methamphetamine regimen remained even if the duration between exposures was increased to 30 days, thus suggesting that the refractoriness induced by the current dosing paradigm was long-lasting and mechanistically distinct from that underlying the temporary tolerance/refractoriness caused by pretreatment with escalating doses of methamphetamine.
Another novel finding of the present study that supports the conclusion of a distinct mechanism is that the methamphetamine “challenge” treatment also did not have an acute (e.g., within 1 hour) effect on striatal plasmalemmal or vesicular dopamine uptake in rats that had been exposed previously to a neurotoxic regimen of methamphetamine. These data stand in contrast to findings that pretreatment with an escalating-dose, methamphetamine-pretreatment paradigm did not alter the methamphetamine-induced decrease in dopamine transporter activity, and only attenuated the methamphetamine-induced decrease in VMAT-2 activity, as assessed 1 hour after a neurotoxic “challenge” regimen (Johnson-Davis et al, 2004). A mechanistic difference between these two types of tolerance-/resistance-inducing paradigms is also suggested by findings of differential microglial responses afforded by these different treatment regimens reported by Thomas and Kuhn (2005). Taken together with data described above, the findings involving the acute effects of methamphetamine on dopamine transporter and VMAT-2 support the possibility that the rapid and long-term responses by nigrostriatal dopamine neurons to methamphetamine treatments may be linked, as both appeared to be eliminated by pretreatment with a “neurotoxic” regimen of methamphetamine. Further research is needed to examine this possibility.
In summary, the present experiments demonstrate that animals pretreated with high-doses of methamphetamine do not show significant alterations in dopamine transporter or VMAT-2 functions and/or dopamine levels in the striatum when exposed to a subsequent neurotoxic methamphetamine challenge, as assessed acutely (1 hour) or 7 days after the second exposure. These findings demonstrate the novel features of the refractoriness-inducing treatment paradigm examined by the present studies and by Thomas and Kuhn (2005) and suggest distinct mechanisms for the acute and long-term responses of this model vs. those associated with a pretreatment consisting of escalating methamphetamine doses (e.g., Danaceau et al., 2007). From these data, one can speculate that the dopaminergic neurons that are seemingly unaffected by the challenge administration used in the present studies are refractory either due to an inherent protective property of the surviving cells in this neuronal pool, as a consequence of protective mechanisms induced by exposure to the methamphetamine neurotoxic pretreatment, or as a consequence of impaired activation of postsynaptic elements in the striatum. Studying these alternatives may help to determine the specific mechanisms contributing to both the vulnerability and resistance to the acute and chronic effects of neurotoxic doses of methamphetamine. Identification of such defensive mechanisms should provide important understanding as to the functioning and vulnerabilities of striatal dopaminergic systems.
Acknowledgements
This work was supported by grants DA 00869, DA 04222, DA 13367, DA 11389, DA 019447, and DA 00378.
References
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: Comparison of escalating and single-day dosing regimens. Neuropsychopharm. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytic. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J. Neurochem. 2000;74:2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J. Pharmacol. Exp. Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol. Biochem. Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced arc mRNA expression in identified striatal efferent neurons. Neurotoxicity Research. doi: 10.1007/BF03033855. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur. J. Pharmacol. 2007;559:46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J. Pharmacol. Exp. Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castañeda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol. Biochem. Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J. Clin. Exp. Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles P-AH, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J. Neurochem. (epub ahead of print) 2008 doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hogan KA, Staal RG, Sonsalla PK. Analysis of VMAT2 binding after methamphetamine or MPTP treatment: disparity between homogenates and vesicle preparations. J. Neurochem. 2000;74:2217–2220. doi: 10.1046/j.1471-4159.2000.0742217.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–1378. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur. J. Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Hanson GR, Keefe KA. Long-term post-synaptic consequences of methamphetamine on preprotachykinin mRNA expression. J. Neurochem. 2002;82:1472–1479. doi: 10.1046/j.1471-4159.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Truong JG, Fleckenstein AE, Wilkins DG. Alterations in vesicular dopamine uptake contribute to tolerance to the neurotoxic effects of methamphetamine. J. Pharmacol. Exp. Ther. 2004;309:578–586. doi: 10.1124/jpet.103.062695. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J. Neuropsychiatry Clin. Neurosci. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur. J. Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur. J. Pharmacol. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J. Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palmero M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, McPherson RJ, Marshall JF. Excitotoxic striatal lesions protect against subsequent methamphetamine-induced dopamine depletions. J. Pharmacol. Exp. Ther. 1994;269:1319–1325. [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, Fleckenstein AE. Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur. J. Pharmacol. 2002;449:71–74. doi: 10.1016/s0014-2999(02)01985-4. [DOI] [PubMed] [Google Scholar]
- Schröder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sonsalla PK, Hanson GR, Peat MA, Gibb JW. Methamphetamine-induced depression of monoamine synthesis in the rat: development of tolerance. J. Neurochem. 1985;44:852–855. doi: 10.1111/j.1471-4159.1985.tb12893.x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neill ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical proficles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharm. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am. J. Addiction. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Stephans S, Yamamoto B. Methamphetamines pretreatment and the vulnerability of the striatum to methamphetamine neurotoxicity. Neuroscience. 1996;72:593–600. doi: 10.1016/0306-4522(95)00587-0. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J Neurochem. 2008;106:696–705. doi: 10.1111/j.1471-4159.2008.05421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J. Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]