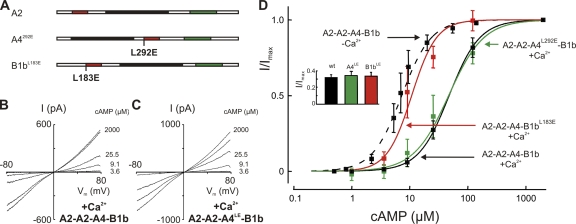
Figure 3.
CNGB1b mediates Ca2+-dependent desensitization. (A) Schematic representation of the three channel subunits indicating the locations of point mutations designed to disable calmodulin-mediated feedback inhibition. (B) I/Vm relations obtained from a patch containing wild-type A2-A2-A4-B1b channels with its endogenous calmodulin recorded in the presence of 20 μM Ca2+. / was 1.31. (C) A similar I/Vm relation obtained from A2-A2-A4L292E-B1b channels indicates that CNA4 does not contribute to desensitization. / was 1.34. (D) Dose–response relations of different subunit combinations tested with endogenous calmodulin. Wild-type A2-A2-A4-B1b channels responded to 20 μM Ca2+ with reduced cAMP sensitivity: K1/2 increased from 7.3 ± 1.7 μM, n = 1.71 ± 0.3 at 0 Ca2+ (dashed line) to 47.8 ± 4.4 μM, n = 1.7 ± 0.3 at 20 μM Ca2+ (solid black line). A similar desensitization was observed with the A2-A2-A4L292E-B1b channel: K1/2 = 46.9 ± 12.0 μM, n = 1.4 ± 0.4 (green), illustrating that the calmodulin-binding site of CNGA4 is not required for desensitization. In contrast, A2-A2-A4-B1bL183E channels did not desensitize in the presence of Ca2+ (K1/2 = 10.8 ± 3.2 μM; n = 2.0 ± 0.8; red), demonstrating that CNGB1b is necessary and sufficient for desensitization in the endogenous complex. (Inset) Controls to document predominant A2-A2-A4-B1b expression in the patches used for these experiments. Measurements were performed with 3.4 μM cAMP. An I/Imax value >0.31 indicates >90% coassembly of A2-A2-A4-B1b channels in the patch. Values were 0.32 ± 0.03 (seven patches) for A2-A2-A4-B1b, 0.34 ± 0.05 (five patches) for A2-A2-A4L292E-B1b, and 0.33 ± 0.04 (five patches) for A2-A2-A4-B1bL183E.