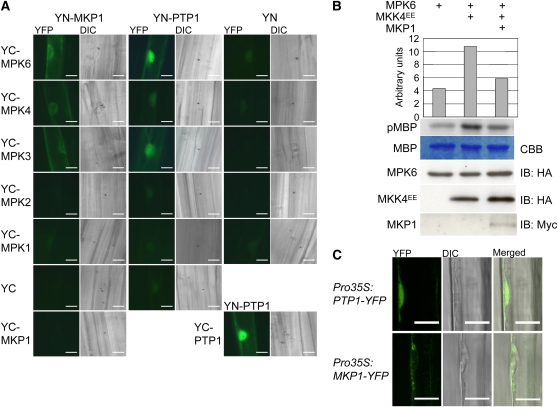
Figure 1.
MKP1 and PTP1 Interact with and Deactivate MPK6, but Differ in Their Subcellular Localization.
(A) BiFC visualization of interactions in transiently transformed mustard hypocotyl cells. YFP signal indicates reconstitution through direct interaction of the tested protein partners. Combinations with YN and YC designate the empty vector controls; YC-MPK1 and YC-MPK2 are shown as negative controls indicating specificity of the detected interactions. The corresponding differential interference contrast (DIC) image is shown for each fluorescence image. Bars = 20 μm.
(B) Arabidopsis protoplasts were transiently transformed with the indicated combinations of HA-MPK6, HA-MKK4EE (constitutively active form), and myc-MKP1, all expressed under the control of the 35S constitutive promoter. Respective MPK6 activities were determined by phosphorylation of the artificial substrate myelin basic protein (MBP) as shown by autoradiography (pMBP) and the corresponding quantification. The MBP panel shows Coomassie blue (CBB) stained loading. Levels of HA-MPK6, HA-MKK4EE, and myc-MKP1 were determined by immunoblot (IB) analysis. A representative experiment out of three comparable repetitions is shown.
(C) MKP1-YFP localizes mainly to the cytoplasm and PTP1-YFP to both the nucleus and cytoplasm. YFP fluorescence in root cells of Arabidopsis seedlings stably expressing cauliflower mosaic virus 35S promoter-driven MKP1-YFP and PTP-YFP fusion constructs as detected with a LSM 510 confocal laser scanning microscope (Zeiss). Bars = 10 μm.