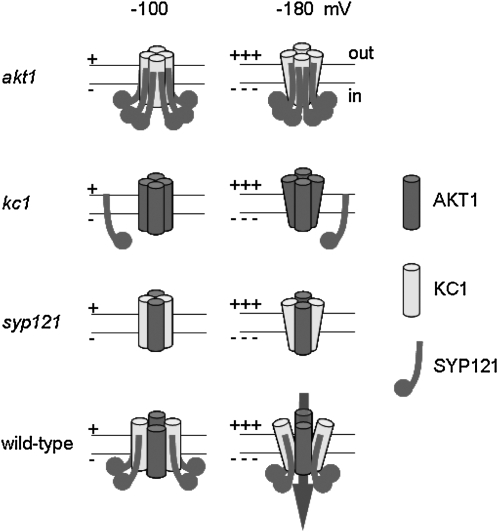
Figure 10.
Tripartite Assembly with SYP121 Determines the Gating of Heteromeric AKT1-KC1 K+ Channels.
Model of channel subunit assembly and gating in the plant based on channel gating characteristics on heterologous expression and in the plant (Figures 3 and 4; see Supplemental Figure 3 online). Eliminating any one of the three proteins AKT1 (akt1), KC1 (kc1) or SYP121 (syp121) prohibits normal gating and K+ flux, either by preventing the assembly of the necessary heteromeric core of AKT1 and KC1 subunits (akt1 and kc1) or by preventing association of SYP121 with the channel core through its binding with KC1 (kc1 and syp121). We assume that AKT1 and KC1 form the core of the channel and its pore, consistent with their structural homologies to other Kv-like K+ channel subunits and the observation that heterologous expression of AKT1 alone and with KC1 yields a current on heterologous expression. The approximate twofold change in the apparent gating charge on coexpression with SYP121 (Figure 3; see also Supplemental Figure 3 online) points to profound changes in the conformation of the channel. Thus, assembly of the heteromeric AKT1-KC1 channel core and binding of at least two SYP121 proteins to each KC1 subunit (wild type) gives a channel gate with native characteristics, underpinning its physiological voltage dependence and K+ flux (arrow).