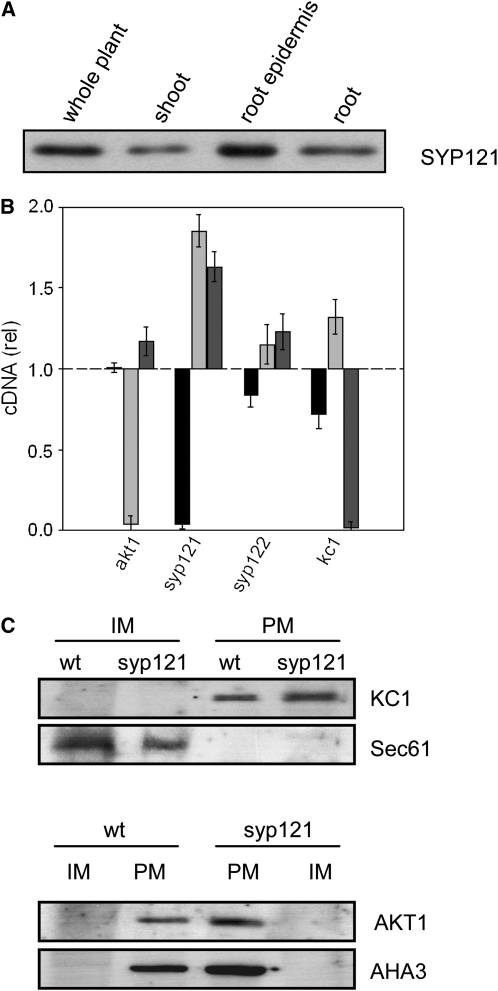
Figure 6.
SNARE and K+ Channel Transcription and Expression in Arabidopsis.
(A) The SNARE SYP121 is expressed strongly in the Arabidopsis root and root epidermis. Protein gel blot analysis of total proteins (10 μg/lane) extracted from whole Arabidopsis, shoot, root, and root epidermis and probed with anti-SYP121 primary antibody. All four lanes yielded a single band close to 37 kD, corresponding to SYP121 (Tyrrell et al., 2007) and consistent with the expression patterns for AKT1 and KC1 (Birnbaum et al., 2003).
(B) Quantification of SYP121 and K+ channel transcripts in the wild type and mutant Arabidopsis. Real-time PCR of SYP121 (black bars), AKT1 (light-gray bars), and KC1 (dark-gray bars) transcript levels in each of the mutant lines syp121-1, syp122-1, akt1-1, and kc1-1 after standardization on ACT2 transcript levels. Data normalized to the corresponding amplification yields in the wild type and are means ± se from three independent experiments. No appreciable decrease was evident for the K+ channel subunits in either of the SNARE mutant or the complementary K+ channel mutant lines. A significant increase (P < 0.05) in relative transcript level was evident in the kc1 and akt1 mutants for AKT1 and KC1 genes, respectively.
(C) KC1 K+ channel localization to the plasma membrane is unaffected in syp121-1 mutant Arabidopsis. Protein gel blot analysis of plasma membrane (PM) and inner membrane (IM) fractions separated by two-phase partitioning of microsomal membranes isolated from roots of wild-type and syp121-1 mutant plants. Parallel SDS-PAGE was run of all fractions (1.3 μg protein/lane), and PVDF membranes were probed with polyclonal antibodies to KC1 and AKT1 (see Supplemental Figure 6 online) before stripping and reprobing with antibodies to the endoplasmic reticulum Sec61 (Yuasa et al., 2005) as a marker for inner membranes and with polyclonal antibody to the H+-ATPase AHA3 (Pardo and Serrano, 1989) as a marker for the plasma membrane. Protein gel blots were visualized by 125I radiotracer phosphor imaging.