Figure 9.
Tripartite Assembly of SYP121 with AKT1 Requires the KC1 Subunit as a Bridge.
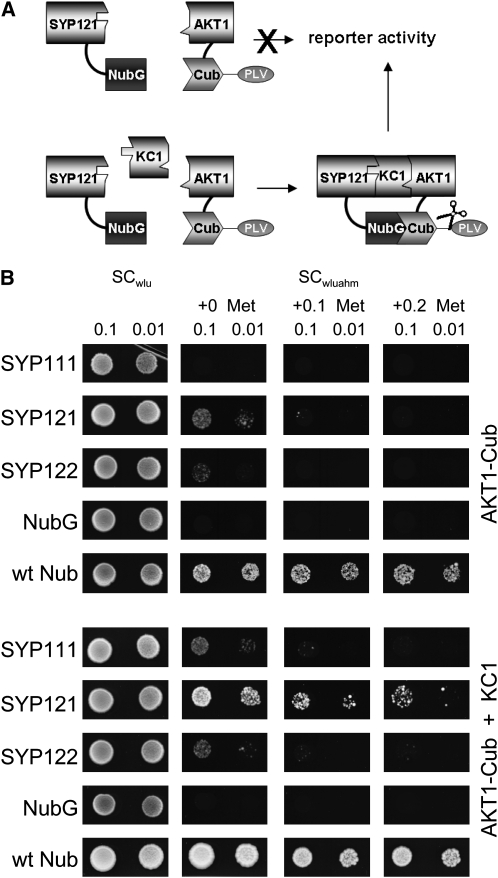
(A) Concept of the yeast SUB assay for formation of a tripartite protein assembly. By analogy with the mating-based split-ubiquitin approach, the SUB assay depends on protein interaction to bring together the two halves of the ubiquitin protein, which leads to cleavage of the VP16 transactivator (PLV) and activation of the reporter gene. In this case, however, assembly of the proteins carrying the split-ubiquitin moieties depends on inclusion of the third protein (KC1) component.
(B) Yeast split-ubiquitin assay for interaction after transformation with AKT1-Cub, the Nub-X fusion constructs of SYP121, SYP122, and SYP111, with (bottom) and without (top) coexpression of KC1. Controls (negative, NubG; positive, wtNub) are included and transformed yeast are spotted (top to bottom) on SC medium without Trp, Leu, and Ura (SCwlu) to verify crossing. Yeast growth on SC without Trp, Leu, Ura, Ade, His, and Met (SCwluahm) was used to verify Ade- and His-independent growth (second panel), and the addition of 0.1 and 0.2 mM Met was used to verify interaction at lower AKT1-Cub expression levels. Serial dilutions (0.1 and 0.01) as indicated for each frame. Note yeast growth on Met only with KC1 coexpression. Protein expression was verified by protein gel blot analysis in each case (data not shown).