Abstract
Study Objectives:
Cheyne-Stokes respirations occur in 40% of patients with heart failure. Orthopnea is a cardinal symptom of heart failure and may affect the patient's sleeping angle. The objective of this study was to assess the respiratory and hemodynamic response to sleeping angle in a group of subjects with stable heart failure.
Design:
Twenty-five patients underwent overnight polysomnography with simultaneous and continuous impedance cardiographic monitoring. Sleeping polysomnographic and impedance cardiographic data were recorded.
Setting:
The study was conducted in a sleep center.
Patients:
All 25 patients had clinically stable heart failure and left ventricular ejection fractions < 40%.
Interventions:
The patients slept at 0°, 15°, 30°, and 45° in random order.
Measurements and Results:
Seventeen patients had Cheyne-Stokes apneas (index > 5/h) and 23 patients had hypopneas (index > 5/h). The hypopnea index showed no response to sleeping angle. The Cheyne-Stokes apnea index decreased with increasing sleeping angle (P < 0.001). This effect was seen only during supine sleep and non-rapid eye movement sleep and was absent in non-supine sleep, rapid eye movement sleep, and during periods of wakefulness. Thoracic fluid content index and left ventricular hemodynamics measured by impedance cardiography showed no response to sleeping angle.
Conclusions:
Changing the heart failure patient's sleeping angle from 0° to 45° results in a significant decrease in Cheyne-Stokes apneas. This decrease occurs on a constant base of hypopneas. The changes in Cheyne-Stokes apneas are not related to changes in lung congestion and left ventricular hemodynamics.
Citation:
Soll BAG; Yeo KK; Davis JW; Seto TB; Schatz IJ; Shen EN. The effect of posture on Cheyne-Stokes respirations and hemodynamics in patients with heart failure. SLEEP 2009;32(11):1499-1506.
Keywords: Body position, polysomnography, impedance cardiography, Cheyne-Stokes respirations, heart failure
HEART FAILURE IS A SERIOUS DISORDER THAT AFFECTS 4,700,000 PEOPLE IN THE UNITED STATES.1 DESPITE ADVANCES IN THERAPY, MORBIDITY AND mortality remain high. Cheyne-Stokes (CS) respirations have been observed in 40% of patients with heart failure 2,3 and are felt to be an independent risk factor for death in these patients.4,5 Further research is needed to better understand the pathophysiology of CS respirations and to identify possible modifying factors that may, in turn, impact heart failure morbidity and mortality.
Orthopnea is a cardinal symptom of heart failure and may affect the patient's sleeping angle. In spite of this, not much is known about the effect of sleeping angle on CS respirations and hemodynamics. In 1958, Altschule studied 6 waking subjects with heart failure and found that changing the patient's posture from 60° to 0° caused or exacerbated periodic breathing.6 Altschule studied a heterogeneous small group of awake subjects. The aim of this investigation is to study the response to posture of a larger group of well-described sleeping subjects using modern noninvasive methods of assessing respiratory and cardiac function.
METHODS
Patient Selection
This study is a prospective, single-blinded study with each individual serving as his or her own control. It was approved by the Institutional Review Boards of the Queen's Medical Center, the University of Hawaii, and Hawaii Pacific Health (all in Honolulu, HI). All subjects provided informed consent.
Stable subjects with clinical heart failure were recruited by direct referral, fliers, and advertisements. All subjects were required to have an echocardiographic left ventricular ejection fraction of less than 40%. Exclusion criteria included acute cardiac or pulmonary disease within 3 months of testing, paced rhythm, complete bundle branch block, primary valvular heart disease, active recreational drug or excessive alcohol use within 3 months of testing, chronic hypnotic use, known obstructive sleep apnea on continuous positive airway pressure, home oxygen use, chronic obstructive lung disease (FEV1/FVC < 70%), known restrictive lung disease, theophylline use, significant renal or liver impairment, and pregnancy.
Patients visited the sleep laboratory twice. On the first visit, informed consent was obtained. A detailed questionnaire was completed that included demographic information as well as a medical and sleep history. A physical examination and spirometry were performed, and blood chemistries were measured. Echocardiograms and electrocardiograms from the referring physicians were reviewed. All patients who met the inclusion criteria were willing to sleep at angles 0° to 45°.
Polysomnography
Each patient underwent overnight polysomnography. The following parameters were recorded: electroencephalogram (C3A2, C4A1, O1C3, O2C4), electrooculogram, submental electromyogram, anterior tibialis electromyogram, electrocardiogram, nasal pressure (Pro Tech PTAFlite pressure transducer airflow sensor, Pro-tech services, Mukilteo, WA, USA), chest and abdominal effort and sum measurements (Pro Tech Synchrony Summing Amplifier, Pro-tech services, Mukilteo, WA, USA) and oxygen saturation (SpO2) using a finger probe (Nellcor, N-200, Pleasanton, CA).7–9
Patients fell asleep at their sleeping angle of comfort. After 30 minutes of sleep, they spent approximately 90 minutes at positions 1, 2, 3, and 4. Seven patients spent 60 minutes at each position because of a delayed initial sleep latency. Sleeping angles of 0°, 15°, 30°, and 45° were assigned to each position in random order.
All patients slept on a Big Boy Bed (Dewert USA Motorized Systems, Frederick, MD). A rheostat (Tenma Variable Auto Transformer, 72-110, Centerville, OH) was used to power down the bed motor to 12 volts. This resulted in slow smooth head-of-bed movement. A laser level (Robotoolz, Mountain View, CA) was attached to the right side of the bed frame. Using an industrial protractor 0°, 15°, 30°, and 45° angles were generated, and the laser was used to calibrate a yardstick that was vertically fastened to the headboard bracket on the right side of the bed. Sleeping angles were adjusted by the nighttime technician from the monitoring room using pendant bed controls and a 45-foot extension cable. The angles were verified by direct vision using the in-room video-monitoring system. Glow sticks (Neon Glo, Shenzhen City, China) were attached to the calibrated yardstick at each of the 4 angle marks for easy identification (Figure 1).
Figure 1.
Big Boy bed, laser level (arrow 1), and calibrated yard stick (arrow 2) with glow sticks (arrow 3).
Sleep stages were scored using standard Rechtschaffen and Kales criteria.10 Ipsilateral occipital leads, in-line analog amplifiers and filters (Model 12 Neurodata Acquistion System, Grass Technologies, West Warwick, RI), and unique workspaces were used to minimize the interference from the impedance cardiography (ICG).
Acquired data were passed from the analog amplifiers through a multiplexor (Lab Master AD/SM, Scientific Solutions, Mentor, OH) to an analog-to-digital converter (Sandman, Nellcor Puritan Bennett [Melville] Ltd, Kanata, ON, Canada) and then stored using Sandman Elite Software 7.1(Sandman). A unique report software package was used to separate the data into the run-in period and positions 1, 2, 3, and 4. Each polysomnogram was scored by a single registered sleep technologist (N. Morin, Sleep Strategies Inc, Ottawa, ON, Canada) and reread by a board-certified sleep specialist (B. Soll). The nighttime technician and the patient were the only persons who knew the assigned sleeping angles; the scoring technologist and the investigators were blinded to the sleeping-angle assignments.
Modifications of the usual clinical standards for respiratory scoring were made. The ICG current produced an electroencephalographic artifact. This was minimized by using the methods described above. Sleep staging was possible, but reliable recognition of arousals in some patients remained a problem. In addition, low-flow pulse-oximetry signals were seen in some of our subjects who had very low cardiac output. This resulted in periods of “bad data” on the oximetry channel. For these 2 reasons, arousals and desaturations were eliminated as a requirement to score an event. Respiratory events were defined as absent if any index was less than 5 events per hour at any sleeping angle. An apnea was defined as cessation of nasal pressure for at least 10 seconds. Hypopnea was defined as a reduction in airflow measured by nasal pressure of greater than 50% for at least 10 seconds. An obstructive apnea was defined as an apnea associated with thoracic and abdominal excursions that were 180° out of phase and a sum channel that was flat or reduced from baseline. A central apnea was scored when the apnea was associated with thoracic, abdominal and sum channels that were flat. CS apnea was defined as at least 3 consecutive cycles of waxing and waning respirations that included a central apnea.11 Flow limitation was identified as well. Respiratory events that occurred during periods of wakefulness were also scored. If CS apneas or hypopneas started in sleep and extended into wakefulness, at least 3 consecutive waking cycles had to occur to be scored.
Sleeping angle or posture refers to the angle of the head of the bed. A foot board was used to keep the patient's hips at the breakpoint of the bed. Sleeping position refers to supine and nonsupine sleep. Nonsupine sleep was defined as sleep occurring in the right or left lateral position. Subjects were asked to not sleep prone. Sleeping angles and positions were validated by the scorers using the video recording. If supine or nonsupine sleep was absent at any given sleeping angle, supine or nonsupine respiratory data from that angle were treated as missing, and the corresponding index was not calculated.
Impedance Cardiography
ICG using the BioZ system (Cardiodynamics, San Diego, CA) was used to monitor cardiac function. The BioZ system was treated as an external device. During calibration, the internal clocks of the Sandman and the BioZ systems were synchronized. The BioZ was turned on, with the onset of position 1. Cardiac function was recorded continuously and simultaneously with the polysomnogram. After each overnight polysomnogram, the ICG data were downloaded to a computer and stored. The following parameters were calculated for each subject at each sleeping angle: stroke index, cardiac index, preejection period, left ventricular ejection time, systolic time ratio (preejection period/left ventricular ejection time), velocity index (peak velocity of blood in the aorta), acceleration index (initial acceleration of blood in the aorta), and thoracic fluid content (TFC).
The BioZ System applies low-amplitude, high-frequency, constant current to the patient's chest and neck and measures both baseline impedance and dynamic impedance changes during the cardiac cycle.12 Dynamic impedance is determined primarily by blood-volume changes in the aorta during each heartbeat. Both baseline impedance and dynamic impedance are used to calculate hemodynamic parameters. As fluid increases in the chest, baseline impedance decreases. As fluid decreases in the chest, baseline impedance increases. TFC is the inverse of the baseline impedance (1/baseline impedance). As fluid decreases, TFC decreases, and, as fluid increases, TFC increases.12–14 To compare one patient with another, TFC index (TFCI; thoracic fluid content/ body surface area) was computed.
Data Analysis
Using a unique software program and the synchronized event windows from the polysomnogram, the ICG data were separated into sleeping and waking data for each position. Data during time out of bed were eliminated. The ICG data were then averaged for each position. The polysomnographic data were also separated into sleeping and waking data and averaged for each position. Then, the randomization code was broken, and appropriate sleeping angles were assigned to each position. These data sets were then analyzed using SAS Enterprise 3.0 (SAS, Inc., Cary, NC).
Using a mixed-model regression procedure, the repeated measures at different angles were identified as clustered within the same patient. We modeled correlations among repeated measures at different angles as well as linear trends by angle and by association with the polysomnographic and ICG variables. Correlations between the angles analyzed were modeled as unstructured, allowing for different correlations between each pair of angles. The analysis was a within-person comparison of outcomes at different angles. As such, patient characteristics that were stable during the changes in sleeping angles should not confound the results. The response of the study variables to increasing “doses” of sleeping angle was expressed as a linear trend or slope. A significant “dose” response was defined as a slope that was different than 0 (P < 0.05). In the demographic comparisons of the group with CS apnea to the group without CS apnea, t tests were used to compare the means of continuous variables, and Fisher exact tests were used to compare proportions. Fisher exact tests were chosen because of the small frequencies for some answers.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Twenty-five subjects were studied. The clinical characteristics, histories, laboratory findings, and medication histories of these subjects are presented in Table 1. Subjects were generally nonobese men older than 60 years of age with mild to moderate clinical heart failure. Their forced vital capacities were at the lower limits of normal and their left ventricular ejection fractions were severely reduced. Paroxysmal nocturnal dyspnea was a common complaint. Subjects complained of orthopnea, habitual snoring, gasping, and choking and observed apnea less frequently. Almost all of the subjects were on optimal medications.
Table 1.
Clinical Characteristics, Laboratory Findings, and Medication History of the Study Population
| Parameter | Results |
|---|---|
| Age, y | 64 ± 12 |
| Men | 20 (80) |
| NYHA Class I – II | 24 (96) |
| BMI, kg/m2 | 28 ± 5.5 |
| Diabetes mellitus | 8 (32) |
| Hypertension | 15 (60) |
| Stroke | 2 (8) |
| Known CAD | 11 (44) |
| History of AF | 10 (40) |
| Defibrillator or pacemaker | 10 (40) |
| Sleep History | |
| Gasping or choking | 6 (24) |
| Habitual snoring | 6 (24) |
| Paroxysmal nocturnal dyspnea | 13 (52) |
| Orthopnea | 3 (12) |
| Observed apnea | 4 (16) |
| ESS score | 7 ± 3.5 |
| Laboratory studies | |
| FEV1 | 80 ± 13 |
| FVC | 82 ± 15 |
| FEV1/FVC | 77 ± 4.4 |
| LVEF, % | 27 ± 7.7 |
| Medication History | |
| β-adrenergic receptor blocking agent | 22 (88) |
| ACE-I or ARB | 23 (92) |
| Loop diuretic | 16 (64) |
| Aspirin | 10 (40) |
| Calcium channel blocker | 1 (4) |
| Aldactone | 5 (20) |
| Digoxin | 12 (48) |
Data are presented as mean ± SD or number (%). NYHA refers to the New York Heart Association; BMI, body mass index; CAD, coronary artery disease; AF, atrial fibrillation; ESS, Epworth Sleepiness Scale; FEV1, Forced expiratory volume in 1 second; FVC, forced vital capacity; LVEF, left ventricular ejection fraction; ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocking agents.
Selected polysomnographic data are shown in Table 2. The linear trends with changes in sleeping angle were not statistically different (P > 0.05) for each variable. Sleep time, sleep efficiency, rapid eye movement (REM) sleep time and percentage of REM sleep were reduced. Sleeping angles were assigned in random order across positions 1, 2, 3, and 4. This resulted in no difference in percentage of REM sleep or REM sleep at each angle.
Table 2.
Selected Polysomnographic Data on All 25 Subjects with 4 Different Angles of the Head of the Bed
| Variable | Angle of the head of the bed, degrees |
|||
|---|---|---|---|---|
| 0 | 15 | 30 | 45 | |
| SOL, min | 5.4 ± 13.9 | 6.5 ± 14.4 | 4.3 ± 4.3 | 8.0 ± 18.6 |
| TST, min | 58.2 ± 26.6 | 60.7 ± 21.6 | 62.6 ± 22.0 | 49.7 ± 23.6 |
| SE, % | 68.3 ± 26.4 | 71.2 ± 24.3 | 74.9 ± 21.5 | 64.7 ± 26.4 |
| REM sleep | ||||
| Minutes | 6.2 ± 8.13 | 7.6 ± 10.4 | 6.1 ± 10.1 | 4.1 ± 6.9 |
| % | 9.4 ± 15.0 | 10.2 ± 13.2 | 9.0 ± 14.7 | 7.3 ± 11.6 |
| Sao2, % | ||||
| Mean | 95.3 ± 1.5 | 95.2 ± 2.2 | 95.7 ± 1.9 | 95.6 ± 1.4 |
| Minimum | 85.0 ± 4.4 | 85.4 ± 5.3 | 86.9 ± 4.8 | 87.0 ± 4.3 |
Data are presented as mean ± SD. SOL refers to sleep-onset latency; TST, total sleep time; SE, sleep efficiency; REM, rapid eye movement; SaO2, oxygen saturation.
Two patients did not tolerate a sleeping angle (one patient at 0° and another patient at 45°). Three patients were unable to sleep at 45°. Data from these 5 angles were treated as missing, and data from the remaining 95 angles were analyzed.
Obstructive apnea, mixed apnea, and pure central apnea were all measured at an index of fewer than 5 events per hour for each angle for all 25 patients.
Seventeen of the 25 patients had CS apneas (CS apnea index of > 5/h for at least 1 sleeping angle), and 23 of the 25 patients had hypopneas (hypopnea index of > 5/h for at least 1 sleeping angle). All of the 17 patients with CS apneas had hypopneas as well. Six of the 23 patients with hypopneas had no CS apneas. Two patients had no CS apneas or hypopneas (index < 5/h for each angle).
Changes in the respiratory indexes by sleeping angle are shown in Table 3. The apnea-hypopnea index decreased with increasing sleeping angle but did not reach significance (slope = −0.12; 95% confidence interval [CI]: −0.36, −0.12). Hypopneas showed no change with sleeping angle (slope = 0.024, 95% CI: 0.15, 0.20). CS apneas, on the other hand, showed a significant decrease with increasing sleeping angles (slope = −0.16; 95% CI: −0.22, −0.10). Examination of the raw data revealed that these changes occurred immediately after the adjustment of the sleeping angle. Supine CS apneas changed significantly with sleeping angle (slope = −0.30, 95% CI: −0.40, −0.20) but nonsupine CS apneas did not (because of the small number of subjects, especially at 45°, the model failed to converge with the unstructured covariance, and a slope could not be generated). The nonsupine CS apnea index was less than the supine CS apnea index at 0° but did not reach significance. As expected, the REM CS apnea index was lower than the NREM CS apnea index. NREM CS apneas changed with sleeping angle (slope = −0.13; 95% CI: −0.21, −0.05) and REM CS apneas did not (because of the small number of subjects, especially at 30°, the model failed to converge with the unstructured covariance, and a slope could not be generated). Waking CS apneas did not change significantly with sleeping angle (slope = −0.05; 95% CI: −0.17, 0.08). Inspiratory flow limitation of varying degrees was seen with many of our subjects. Careful review of the raw data revealed no consistent change in flow limitation with decreasing sleeping angle.
Table 3.
Respiratory Indexes by Angle of the Head of the Bed in Degrees
| Index | 0° | 15° | 30° | 45° | P value |
|---|---|---|---|---|---|
| Apnea-hypopnea | 34.7 ± 30.0 | 29.7 ± 24.2 | 28.4 ± 23.6 | 23.2 ± 23.7 | > 0.05 |
| Hypopnea | 15.3 ± 13.8 | 16.1 ± 9.9 | 17.2 ± 14.3 | 15.9 ± 19.0 | > 0.05 |
| CS apnea | |||||
| Total | 19.4 ± 28.2 | 13.6 ± 24.7 | 11.2 ± 17.6 | 7.3 ± 15.8 | <0.001 |
| Supine | 34.3 ± 27.8 | 19.9 ± 32.4 | 16.6 ± 19.3 | 8.2 ± 13.8 | <0.001 |
| Nonsupine | 25.4 ± 42.9 | 14.6 ± 23.1 | 30.1 ± 59.7 | 29.2 ± 41.2 | 0.54 |
| NREM | 27.8 ± 31.2 | 19.7 ± 28.4 | 15.5 ± 19.2 | 11.2 ± 19.6 | 0.005 |
| REM | 7.52 ± 14.6 | 7.94 ± 21.1 | 0.00 | 6.99 ± 17.3 | NS |
| Waking | 16.9 ± 27.5 | 11.5 ± 20.0 | 15.5 ± 22 5 | 9.9 ± 16.7 | > 0.05 |
Data are shown as mean ± SD for 23 subjects for the apnea-hypopnea and hypopnea indexes and 17 subjects for all other indexes. CS refers to Cheyne-Stokes respirations; NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep.
Subjects were then divided into 2 groups for analysis: those with CS apnea (17 subjects) and those without CS apnea (8 subjects). The 17 patients with CS apneas are broken down in Table 4. CS apnea indexes are given for each subject and each angle. The individual dose response or slope is also noted. Each position is coded by sleeping position. Positions with REM sleep are in bold. The last position of the night is underlined. Eleven subjects had the expected negative slope, and 6 did not. The variance can be explained by REM, “end-of-night,” and sleeping-position effects. Subject's 6, 11, and 20 positive slopes can be explained by an end-of-night boost in their CS apnea indexes. Subject 23 had a low index at 0° during REM sleep in the nonsupine position and an end-of-night boost at 15°. Subject 12 slept nonsupine at 0° and had a mix of hypopneas and apneas. At 15°, he slept for 14 of the 60 minutes in the nonsupine position and had more CS apneas and fewer hypopneas. Finally, at 30° and 45°, he slept supine and had almost exclusively CS apneas. Subject 8 slept in the nonsupine position at 0° for only 7 of the 90 minutes. In that position, he had a CS apnea index of less than 5 and large numbers of hypopneas. These hypopneas became CS apneas when he assumed the supine position at other angles. Subject 15's index at 0° is an outlier. This subject had continuous CS apnea, marked sleep fragmentation, and a mean total cycle length of 46.6 seconds.
Table 4.
Cheyne-Stokes Apnea Index by Degree for Each of the 17 Subjects with Cheyne-Stokes Apnea
| Subject | 0° | 15° | 30° | 45° | Slope |
|---|---|---|---|---|---|
| 1 | S 16 | S 7.9 | S 8.5 | S 0 | −0.32 |
| 2 | B 9.6 | S 0 | S 0 | S 0 | −0.2 |
| 3 | N 67.4 | N 65.6 | N 46.9 | N 58.3 | −0.31 |
| 5 | S 45.9 | B 8.6 | B 0 | S 0 | −0.89 |
| 6 | B 0 | B 0 | B 19.5 | B 0 | 0.2 |
| 7 | S 34.3 | B 0 | S 0 | S 0 | −0.67 |
| 8 | B 0 | S 56.3 | S 34.7 | S 32.2 | 0.45 |
| 9 | B 5.6 | B 0 | B 0 | S 0 | −0.11 |
| 11 | S 0 | S 0 | S 20 | S 0 | 0.17 |
| 12 | B 42.4 | N 33.7 | S 50.5 | S 40.3 | 0.07 |
| 14 | S 20.9 | S 0 | S 0 | S 0 | −0.42 |
| 15 | S 107.4 | S 68.4 | S 31.8 | −2.5 | |
| 18 | B 7.4 | B 0 | S 0 | S 0 | −0.15 |
| 20 | N 0 | N 0 | S 5.3 | 0.18 | |
| 23 | N 0 | N 11.1 | B 5.6 | 0.19 | |
| 24 | S 60 | B 74.3 | S 51.7 | −0.28 | |
| 25 | S 41.1 | B 0 | S 0 | −1.3 | |
| Mean | −0.16 |
S, Supine; N, Nonsupine; B, Sp/Nonsp
Slope refers to individual dose response to posture; blank cell, position not tolerated or no sleep; bold, position with REM sleep. Underlined, last position of the night.
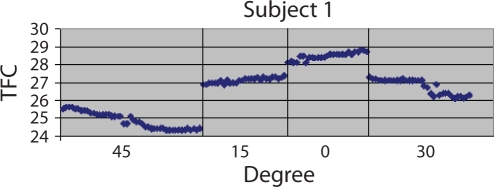
Mean left ventricular hemodynamic and TFCI measurements by ICG on the 17 patients with CS apnea are reported in Table 5. Small changes in each parameter were seen with changes in sleeping angle. However, no significant linear trends were seen with any of the parameters. Figure 2 shows an example of these small changes in Subject 1. TFC changes abruptly with adjustments in sleeping angle. A gradual drift over each period is also seen as thoracic fluid and lung volume come into equilibrium. The y axis is amplified to demonstrate these small changes. When the ICG values of the group with CS apnea were compared with the ICG values of the group without CS apnea, no significant differences in any of the parameters at each angle were seen except for TFCI. The TFCI was higher across all angles in the group without CS apnea, compared with the group with CS apnea (P = 0.05).
Table 5.
Left Ventricular Hemodynamic and Thoracic Fluid Content Index Measurements by Impedance Cardiography of the 17 Patients with Cheyne-Stokes Apnea
| Normal | 0° | 15° | 30° | 45° | |
|---|---|---|---|---|---|
| CI, L·min−1·m−2 | 2.5-4.2 | 1.88 ± 0.48 | 2.04 ± 0.77 | 1.92 ± 0.50 | 1.88 ± 0.46 |
| SI, mL·beat·m−2 | 35-65 | 29.2 ± 9.63 | 31.1 ± 12.9 | 30.0 ± 9.85 | 29.1 ± 8.65 |
| HR, beats·min−1 | 60-100 | 68.0 ± 13.1 | 68.1 ± 10.7 | 66.9 ± 11.3 | 66.2 ± 10.9 |
| PEP, sec | 0.05-0.12 | 0.13 ± 0.03 | 0.13 ± 0.02 | 0.13 ± 0.03 | 0.14 ± 0.02 |
| LVET, sec | 0.25-0.35 | 0.28 ± 0.04 | 0.28 ± 0.04 | 0.28 ± 0.04 | 0.27 ± 0.03 |
| STR | 0.30-0.50 | 0.50 ± 0.15 | 0.52 ± 0.16 | 0.50 ± 0.15 | 0.56 ± 0.13 |
| VI, mL·1000−1·sec−2 | 33-65 | 24.7 ± 8.85 | 30.8 ± 24.5 | 25.6 ± 8.82 | 26.3 ± 8.86 |
| AI, mL·100-1·sec−2a | 70-150 | 44.2 ± 14.0 | 56.5 ± 42.5 | 48.8 ± 24.3 | 47.5 ± 17.1 |
| TFCI, kOhm-1m−2b | 17.2 ± 2.7 | 17.0 ± 3.2 | 17.2 ± 3.3 | 17.7 ± 3.7 |
Data are shown as mean ± SD. CI refers to cardiac index; SI, stroke index; HR, heart rate; PEP, preejection period; LVET, left ventricular ejection time; STR, systolic time ratio (PEP/LVET); VI, velocity index—the peak velocity of blood in the aorta; AI, acceleration index—the initial acceleration of blood in the aorta; TFCI, thoracic fluid content index.
The normal value is shown for men; for women, the value is 90-170 mL·100−1·sec−2.
The normal value for TFC is 30-50 kOhm−1 for men; for women, the value is 21-37 kOhm−1.
Figure 2.
Thoracic fluid content (TFC) by sleeping angle in Subject 1. The y axis is amplified to show the small changes that occur.
Both groups were also examined for differences in medical history, sleep history, medication use, physical findings, laboratory values, spirometry results, and echocardiogram results. No significant differences were seen.
DISCUSSION
In our group of subjects with stable heart failure, changing the sleeping angle from 45° to 0° caused or exacerbated CS apneas. Hypopneas, on the other hand, showed no response to sleeping angle. Supine and NREM CS apneas showed a significant response to sleeping angle and nonsupine, REM, and waking CS apneas did not. Left ventricular hemodynamics and TFCI measured by ICG remained constant throughout the night in spite of changes in posture.
CS respirations are felt to originate from a central apnea that is the result of hyperventilation and a reduction of the pCO2 below the apneic threshold.15,16 This hyperventilation is, in turn, thought to be secondary to stimulation of vagal irritant receptors by pulmonary congestion.17,18 This hypothesis is consistent with Altschule's observations that lowering the head of the bed from 60° to 0° caused or exacerbated periodic breathing in his waking subjects.6 One would expect the lungs to be more congested at 0° because of increased venous return.
A higher TFCI at 0° and a lower value at 45° were expected in this study. However, no significant change in TFCI was seen with changes in the sleeping angle. Furthermore, the TFCI in the group without CS apnea was significantly higher than the group with CS apnea.
There are several possible explanations for these findings. CS respirations are felt to occur in patients with heart failure with unstable closed-loop chemical control of ventilation. This loss of stability is felt to be due to the slow circulation time between the lungs and chemoreceptors and enhanced loop gain.15,19 It is possible, in this destabilized system, that the small statistically insignificant changes that were seen in TFCI were somehow amplified and resulted in significant changes in CS apnea.
TFC is a relatively new clinical parameter. Those unfamiliar with it might be tempted to consider it an insensitive surrogate of lung congestion. However, there are several recent studies that suggest that this is not the case. Van de Water found TFC to be superior to traditional hemodynamic and gas-transfer parameters in trending chest fluid changes during lactated Ringers infusion in dogs.20 In the PREDICT study, Packer et al. followed 212 stable patients with heart failure for 26 weeks. ICG measurements (BioZ ICG Monitor) were made every 2 weeks. TFC, velocity index, and left ventricular ejection time were each found to be independent predictors of death or worsening of heart failure during the 14 days after testing.21 Finally, Yu et al. studied 33 patients with New York Heart Association class III and IV heart failure that were implanted with a special pacemaker that measured and recorded intrathoracic impedance between a right ventricular lead and the pacemaker case. He found that intrathoracic impedance decreased before each admission by an average of 12.3%. In addition, there was an inverse correlation between intrathoracic impedance and pulmonary capillary wedge pressure (r = −0.61, P < 0.001) and between intrathoracic impedance and net fluid loss (r = −0.7, P < 0.001) during hospitalization.22
It is also possible that the supine position and increased venous return resulted in dilation of the left heart but no increase in lung congestion and that this dilation was responsible for the respiratory changes. Tkacova et al. found higher left ventricular end-diastolic and end-systolic volumes in patients with heart failure and CS respirations, compared with those without CS respirations.23 The authors noted that left ventricular filling pressures are not always elevated in patients with left ventricular dilation. Lloyd found that distension of the left atrium in an isolated dog model increased respiratory frequency.24 If this increased frequency lead to hyperventilation, this would predispose to CS apnea. However, Rapaport et al. found no change in end-diastolic and end-systolic volume in his subjects with heart failure when they were tilted from the supine to the upright position.25 The ICG doesn't measure left-heart volumes. It remains unclear what role these volumes played in our patients.
It would appear that changes in lung congestion are not the cause of the changes we observed in the CS apnea index. However, it remains likely that lung congestion is playing a role in generating CS hypopneas.18,26 If that is the case, the TFCI should be higher in a group of subjects with CS hypopneas and lower in a group without CS hypopneas. This should be the topic of a future study.
This study is unique because sleeping angles were changed, and continuous recordings of ICG-derived measures of left ventricular hemodynamics were made. The ICG measures of cardiac output have been studied extensively in waking patients and have been shown to have excellent reproducibility and to correlate well with the thermal dilution and Fick methods.27–30 All of the ICG measures in our subjects with heart failure showed no response to changes in sleeping angle and were stable throughout the night. Our results are similar to those of Rapaport et al.25 They found no significant change in heart rate, stroke volume, and end-systolic and end-diastolic volume when their subjects with heart failure were tilted from the supine to the upright position.
We also found no difference between the ICG measurements in the group with CS apnea and the group without CS apnea. Other authors have reported similar findings using echocardiography and ejection fraction as their measure of left ventricular function.31,32 All of these data suggest that there is no relationship between the changes in the CS apnea index and left ventricular function.
Szollosi et al., Sahlin et al., and Oksenberg et al. demonstrated that CS respirations are less prevalent in patients in the nonsupine position.33–35 Although not statistically significant, our patients also had a lower CS apnea index when they slept in the nonsupine, compared with the supine, position at 0°. Szollosi et al. presented 2 explanations for their findings: gravity-related upper airway instability and decreased lung volume with reductions in pulmonary oxygen stores leading to hyperventilation and loop instability.33,34 Our patients' baseline forced vital capacities were at the lower limits of normal. It could be argued that their resting lung volumes decreased further when their sleeping angles were reduced from 45° to 0°. The ICG findings do not support this conclusion. Decreased thoracic gas volume and increased thoracic fluid both result in a decrease in bioimpedance and an increase in TFCI. Although TFCI has not been validated as a surrogate marker of lung volume, one would expect changes in TFCI if there were significant changes in resting lung volume. As mentioned earlier, TFCI showed no change with sleeping angle. In addition, changes in lung volumes would be expected to result in changes in oxygen saturation. Our patients' saturations were not significantly different at the 4 sleeping angles.
McEvoy et al. showed that obstructive apnea is less prevalent in the upright, compared with the supine, posture in obese patients with OSA.36 Other authors have demonstrated reductions in the size of the pharynx in patients with OSA and, importantly, in normal control subjects when the sleeping angles are changed from the upright to the supine position.37–38 Increases in upper airway resistance have been demonstrated with decreasing tidal volumes in normal subjects with CS respirations.39 Similar findings have been seen in elderly patients with CS respirations.40 Pharyngeal narrowing and occlusion is known to occur in patients with congestive heart failure who have CS apnea41 and in normal subjects with spontaneous and ventilator-induced central apnea.42 The increase in upper airway resistance and occlusion is felt to be due to decreases in neural drive to the pharyngeal muscles. It is of interest that Altschule et al. could ameliorate the periodic breathing in their subjects with heart failure in the horizontal position by “flexing the neck…whereas the head became elevated…without changing the position of the trunk.”6 It is likely that this maneuver extended the head relative to the neck and placed the subjects in the “sniff position,” thus opening their upper airway. This observation is consistent with the work of Walsh et al., who reported a decrease in pharyngeal critical pressure associated with extension of the head.43
In our subjects, CS apneas evolved from hypopneas and then returned again with changes in sleeping position and posture. It is difficult to document these changes because other variables such as end-of-night effect44 and REM sleep impact the prevalence of CS apneas. In addition, at any given angle and position, CS apneas come and go. Gravity-related upper airway instability may be the best explanation for the changes we observed in the CS apnea index. It is of interest that, as the sleeping angle changed from 0° to 45°, our patients' supine CS apnea index dropped significantly, but the nonsupine CS apnea index did not change at all. The lack of change in the nonsupine index may be due to the reduced effect of gravity on the pharynx when its long axis is positioned in the vertical plane. The supine index, on the other hand, would be expected to change more significantly when the short axis is in the vertical plane.45 Further support for upper airway instability is the lack of change in the supine waking CS apnea index with changes in posture. Pharyngeal muscle-dilating activity should increase during periods of wakefulness, thereby decreasing upper airway compliance and reducing the effect of gravity on the upper airway.
Two observations in our study do not support a gravity-related explanation. REM-related CS apneas showed no significant response to changes in sleeping angle. One would expect that the atonia of REM sleep would predispose patients to developing upper airway instability and obstruction. However, the number of apneas in this group was small, and no CS apneas were seen at 30°. Larger number of patients are necessary to determine the effect of posture during REM sleep. Lastly, inspiratory flow limitation was seen in many of our patients but didn't appear to be related to sleeping angle.
There are several limitations to this study. The sample size is relatively small. The study should be repeated in a larger patient population. In addition, our subjects had mild to moderate clinical heart failure and were stable, well compensated, optimally treated, and not obese. Patients with known obstructive sleep apnea on continuous positive airway pressure were excluded. The results may be different in other patient groups. Our study was designed to examine the relationship between ICG measures of left ventricular hemodynamics and lung congestion and CS apneas. Many of our subjects had flow limitation consistent with some element of upper air obstruction. A repeat study with airflow and esophageal pressure monitoring may shed more light on the role that the upper airway plays in these postural and positional changes. Lastly, we have presented evidence that supports ICG technology as a reliable and accurate tool to measure sleeping cardiac function; however, future studies must be done before this technology is used routinely in sleep research and clinical polysomnography.
We have demonstrated that it is possible to collect polysomnographic data and simultaneously monitor cardiac function using ICG technology. We have also shown that changing the patient's sleeping angle from 0° to 45° results in a significant decrease in CS apneas. This decrease occurs on a constant base of hypopneas. It would appear that the changes we observed in CS apneas are not related to changes in lung congestion and left ventricular hemodynamics. They may be related to upper airway instability or possibly to dilation of the left heart that occurs with changes in posture. Our results suggest that future studies of CS respirations should account for sleeping angle and position and should discriminate between CS apneas and hypopneas, since they appear to behave differently.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to acknowledge the following individuals for their invaluable contributions: Administrative support: Carol Agard, Shiela Ludwig; Recruiting: Reden Esquillo, Jung Kim, Tony Magaoay, Ann Cook; Data collection: Melvina Kaliko; Data management: Will Chen.
This study was supported by The Leahi Fund and The Queen Emma Foundation, both of Honolulu, Hawaii, and Research Centers in Minority Institution award, P20 RR011091, from the National Center for Research Resources, National Institutes of Health, and NCRR grant R25 RR019321, “Clinical Research Education and Career Development (CRECD) in Minority Institutions.” Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR/NIH.
The study was performed at the Queens Medical Center, Honolulu, Hawaii.
Cardiodynamics Corporation loaned us a BioZ. They did not participate in the design of the study, analysis of the data or construction of the manuscript.
REFERENCES
- 1.2001 Heart and Stroke Statistical Update. Dallas: American Heart Association; 2000. [Google Scholar]
- 2.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 4.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 5.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 6.Altschule MD, Iglauer A. The effect of position on periodic breathing in chronic cardiac decompensation. N Engl J Med. 1958;259:1064–6. doi: 10.1056/NEJM195811272592204. [DOI] [PubMed] [Google Scholar]
- 7.Flemons WW, Buysee DP, Redline S, et al. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 8.Clark SA, Wilson CR, Satoh M, Pegelow D, Dempsey JA. Assessment of inspiratory flow limitation invasively and noninvasively during sleep. Am J Respir Crit Care Med. 1998;158:713–22. doi: 10.1164/ajrccm.158.3.9708056. [DOI] [PubMed] [Google Scholar]
- 9.Staats BA, Bonekat HW, Harris CD, Offord KP. Chest wall motion in sleep apnea. Am Rev Respir Dis. 1984;130:59–63. doi: 10.1164/arrd.1984.130.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Kales AA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 11.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events, Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 12.Osypka MJ, Bernstein DP. Electrophysiologic principles and theory of stroke volume determination by thoracic electrical bioimpedance. AACN Clin Issues. 1999;10:385–99. doi: 10.1097/00044067-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Luepker RV, Michael JR, Warbasse JR. Transthoracic electrical impedance; quantitative evaluation of a non-invasive measure of thoracic fluid volume. Am Heart J. 1973;85:83–93. doi: 10.1016/0002-8703(73)90529-2. [DOI] [PubMed] [Google Scholar]
- 14.Strobeck JE, Silver MA, Ventura H. Impedance cardiography: noninvasive measurement of cardiac stroke volume and thoracic fluid content. Congest Heart Fail. 2000;6:56–9. doi: 10.1111/j.1527-5299.2000.80144.x. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–54. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 16.Solin P, Roebuck T, Swieca J, Walters EH, Naughton MT. Effects of cardiac dysfunction on non-hypercapnic central sleep apnea. Chest. 1998;113:104–10. doi: 10.1378/chest.113.1.104. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzi-Filho G, Rankin F, Bies I, Bradley TD. Effects of inhaled carbon dioxide and oxygen on cheyne-stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 1999;159:1490–8. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- 18.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–9. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 19.Pinna GD, Maestri R, Mortara A, La Rovere MT, Fanfulla F, Sleight P. Periodic breathing in heart failure patients: testing the hypothesis of instability of the chemoreflex loop. J Appl Physiol. 2000;89:2147–57. doi: 10.1152/jappl.2000.89.6.2147. [DOI] [PubMed] [Google Scholar]
- 20.van de Water JM, Mount BE, Chandra KM, Mitchell BP, Woodruff TA, Dalton ML. TFC (thoracic fluid content): a new parameter for assessment of changes in chest fluid volume. Am Surg. 2005;71:81–6. [PubMed] [Google Scholar]
- 21.Packer M, Abraham WT, Mehra MR, et al. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47:2245–52. doi: 10.1016/j.jacc.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 22.Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–8. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 23.Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med. 1997;156:1549–55. doi: 10.1164/ajrccm.156.5.9612101. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd TC. Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol. 1990;69:1973–80. doi: 10.1152/jappl.1990.69.6.1973. [DOI] [PubMed] [Google Scholar]
- 25.Rapaport E, Wong M, Escobar EE, Martinez G. The effect of upright posture on right ventricular volumes in patients with and without heart failure. Am Heart J. 1966;71:146–52. doi: 10.1016/0002-8703(66)90177-3. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19:37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- 27.Albert NM, Hail MD, Li J, Young JB. Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am J Crit Care. 2004;13:469–79. [PubMed] [Google Scholar]
- 28.Sageman WS, Riffenburgh RH, Spiess BD. Equivalence of bioimpedance and thermodilution in measuring cardiac index after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:8–14. doi: 10.1053/jcan.2002.29635. [DOI] [PubMed] [Google Scholar]
- 29.Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography: the next vital sign technology? Chest. 2003;123:2028–33. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 30.Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Fail. 2003;9:241–50. doi: 10.1111/j.1751-7133.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 31.Vazir A, Hastings PC, Dayer M, et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail. 2007;9:243–50. doi: 10.1016/j.ejheart.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Villa M, Lage E, Quintana E, et al. Prevalence of sleep breathing disorders in outpatients on a heart transplant waiting list. Transplant Proc. 2003;35:1944–5. doi: 10.1016/s0041-1345(03)00655-9. [DOI] [PubMed] [Google Scholar]
- 33.Sahlin C, Svanborg E, Stenlund H, Franklin KA. Cheyne-Stokes respiration and supine dependency. Eur Respir J. 2005;25:829–33. doi: 10.1183/09031936.05.00107904. [DOI] [PubMed] [Google Scholar]
- 34.Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position reduces severity of central sleep apnea / Cheyne-Stokes respiration. Sleep. 2006;29:1045–51. doi: 10.1093/sleep/29.8.1045. [DOI] [PubMed] [Google Scholar]
- 35.Oksenberg A, Arons E, Snir D, Radwan H, Soroker N. Cheyne-Stokes respiration during sleep: a possible effect of body position. Med Sci Monit. 2002;8:CS61–5. [PubMed] [Google Scholar]
- 36.McEvoy RD, Sharp DJ, Thornton AT. The effects of posture on obstructive sleep apnea. Am Rev Respir Dis. 1986;133:662–6. doi: 10.1164/arrd.1986.133.4.662. [DOI] [PubMed] [Google Scholar]
- 37.Battagel JM, Johal A, Smith AM, Kotecha B. Postural variation in oropharyngeal dimensions in subjects with sleep disordered breathing: a cephalometric study. Eur J Orthod. 2002;24:263–76. doi: 10.1093/ejo/24.3.263. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto K, Ozbek MM, Lowe AA, Fleetham JA. Effect of body position on tongue posture in awake patients with obstructive sleep apnoea. Thorax. 1997;52:255–9. doi: 10.1136/thx.52.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Applied Physiol. 1986;61:1438–44. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- 40.Hudgel DW, Hamilton HB. Respiratory muscle activity during sleep-induced periodic breathing in the elderly. J Appl Physiol. 1994;77:2285–90. doi: 10.1152/jappl.1994.77.5.2285. [DOI] [PubMed] [Google Scholar]
- 41.Alex CG, Onal E, Lopata M. Upper airway occlusion during sleep in patients with Cheyne-Stokes respiration. Am Rev Respir Dis. 1986;133:42–5. doi: 10.1164/arrd.1986.133.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 43.Walsh JH, Maddison KJ, Platt PR, Hillman DR, Eastwood PR. Influence of head extension, flexion, and rotation on collapsibility of the passive upper airway. Sleep. 2008;31:1440–7. [PMC free article] [PubMed] [Google Scholar]
- 44.Tkacova R, Niroumand M, Lorenzi-Filho G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure. Circulation. 2001;103:238–43. doi: 10.1161/01.cir.103.2.238. [DOI] [PubMed] [Google Scholar]
- 45.Leiter JC. Upper airway shape: Is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med. 1996;153:894–8. doi: 10.1164/ajrccm.153.3.8630569. [DOI] [PubMed] [Google Scholar]