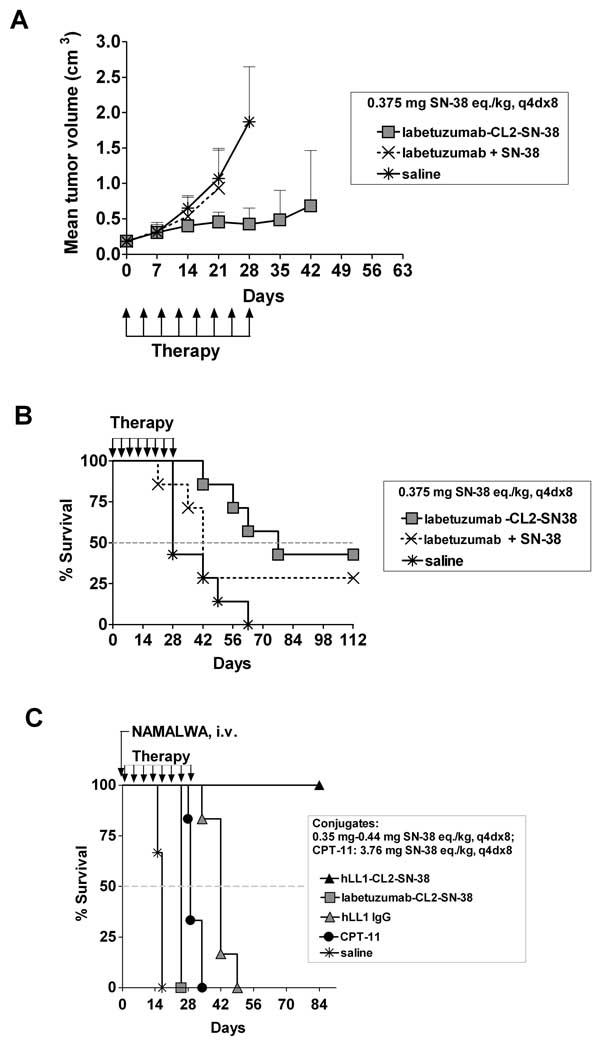
Figure 4.
Therapy of the sc CaPan 1 human pancreatic adenocarcinoma xenograft in nude mice (A&B) and evaluation in a CEACAM5-negative systemic lymphoma model in SCID mice (C). In the sc CaPan 1 model, groups of mice (n = 7) were administered the specific labetuzumab-CL2-SN-38 conjugate at 0.375 mg SN-38 eq./kg, a noncovalent mixture of labetuzumab (25 mg/kg, same as in the conjugate) and SN-38 (0.375 mg/kg, same as in the conjugate), or saline, each at a dose schedule of q4d×8. (A) Plot of mean tumor volume vs. time, and (B) Kaplan-Meier survival plots. (C) Groups of SCID mice (n = 6) were injected i.v. with NAMALWA lymphoma cells, and the treatment was started one day later with the targeting anti-CD74 mAb, hLL1, the targeting hLL1-CL2-SN-38 conjugate, non-targeting hMN-14-CL2-SN-38 conjugate, CPT-11, or saline. The conjugates were administered at 0.35–0.44 mg SN-38 eq./kg, q4d×8; CPT-11 at 3.76 mg SN-38 eq./kg, q4d×8; hLL1 at the equi-protein dose of 25 mg/kg, q4d×8; and saline, q4d×8. Figure shows the Kaplan-Meier survival plots.