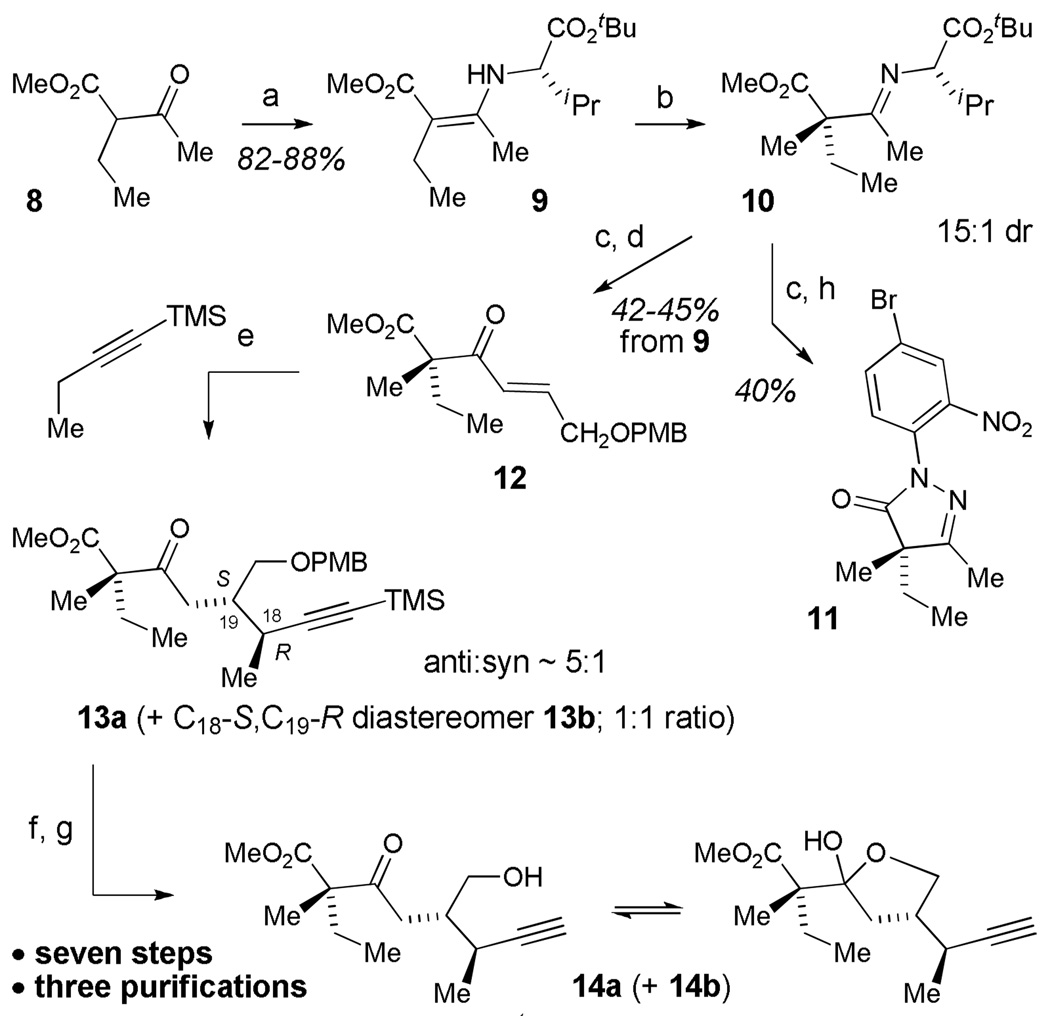
Scheme 2.
Synthesis of alkyne fragmenta
aReagents and conditions: (a) L-tBu-Val-NH2, BF3·Et2O, PhH, reflux (82–88%); (b) LDA, PhMe, −78 °C, 1 h, THF (2.5 equiv.), −78 °C, 3 h, MeI, −78 °C, 17 h; (c) 1 M aq. HCl/THF (1:1), rt, 1 h; (d) TiCl4, THF, 4 ÅMS, 0 °C, 30 min, NEt3, −78 °C, 1 h, PMBOCH2CHO, −78 °C, 1.5 h, rt 1.5 h (42–45%, 3 steps); (e) 1-trimethylsilyl-1-butyne, sBuLi, THF, −78 °C, 2 h, CuBr·SMe2, −78 °C, 1 h, then add 12, −78 °C, 24 h; (f) K2CO3, MeOH, rt, 2 h; (g) DDQ, CH2Cl2/H2O (7:1), rt (70%, 3 steps); (h) 4-Br-2-NO2PhNHNH2·HCl, EtOH, reflux, 2 d (40%, 2 steps).