Abstract
Background
The value of robotics in general surgery may be for advanced minimally invasive procedures. Unlike other specialties, formal fellowship training opportunities for robotic general surgery are few. As a result, most surgeons currently develop robotic skills in practice. Our goal was to determine whether robotic cholecystectomy is a safe and effective bridge to advanced robotics in general surgery.
Methods
Before performing advanced robotic procedures, 2 surgeons completed the Intuitive Surgical da Vinci training course and agreed to work together on all procedures. Clinical surgery began with da Vinci cholecystectomy with a plan to begin advanced procedures after at least 10 cholecystectomies. We performed a retrospective review of our pilot series of robotic cholecystectomies and compared them with contemporaneous laparoscopic controls. The primary outcome was safety, and the secondary outcome was learning curve.
Results
There were 16 procedures in the robotics arm and 20 in the laparoscopic arm. Two complications (da Vinci port-site hernia, transient elevation of liver enzymes) occurred in the robotic arm, whereas only 1 laparoscopic patient (slow to awaken from anesthetic) experienced a complication. None was significant. The mean time required to perform robotic cholecystectomy was significantly longer than laparoscopic surgery (91 v. 41 min, p < 0.001). The mean time to clear the operating room was significantly longer for robotic procedures (14 v. 11 min, p = 0.015). We observed a trend showing longer mean anesthesia time for robotic procedures (23 v. 15 min). Regarding learning curve, the mean operative time needed for the first 3 robotic procedures was longer than for the last 3 (101 v. 80 min); however, this difference was not significant. Since this experience, the team has confidently gone on to perform robotic biliary, pancreatic, gastresophageal, intestinal and colorectal operations.
Conclusion
Robotic cholecystectomy can be performed reliably; however, owing to the significant increase in operating room resources, it cannot be justified for routine use. Our experience, however, demonstrates that robotic cholecystectomy is one means by which general surgeons may gain confidence in performing advanced robotic procedures.
Abstract
Contexte
En chirurgie générale, la robotique peut être valable pour des interventions avancées à effraction minimale. Contrairement à d’autres spécialités, les possibilités de stage (fellowship) structuré en chirurgie générale robotique sont rares. C’est pourquoi la plupart des chirurgiens acquièrent actuellement leurs techniques de robotique dans la pratique. Nous voulions déterminer si la pratique de la cholécystectomie assistée par robotique peut mener efficacement et en toute sécurité à la robotique avancée en chirurgie générale.
Méthodes
Avant de pratiquer des interventions avancées assistées par robotique, 2 chirurgiens ont terminé le cours de formation da Vinci en chirurgie intuitive et ont accepté de travailler ensemble pour toutes leurs interventions. La chirurgie clinique a commencé par la cholécystectomie da Vinci; on prévoyait commencer à pratiquer des interventions avancées après avoir réalisé au moins 10 cholécystectomies. Nous avons procédé à une étude rétrospective de notre série pilote de cholécystectomies assistées par robotique et nous les avons comparées à des interventions témoins pratiquées à la même époque en laparoscopie. La sécurité constitue le premier résultat et la courbe d’apprentissage, le deuxième.
Résultats
Le volet robotique comportait 16 interventions et le volet laparoscopique, 20. Il y a eu 2 complications (hernie de l’orifice da Vinci, élévation transitoire des enzymes hépatiques) dans le volet robotique tandis qu’un seul patient qui a subi une laparoscopie (a pris du temps à se réveiller de l’anesthésie) a eu une complication. Aucune complication n’a été significative. Il a fallu en moyenne beaucoup plus de temps pour pratiquer la cholécystectomie assistée par robotique qu’il en a fallu pour pratiquer la laparoscopie (91 c. 41 min, p < 0,001). Il a fallu en moyenne beaucoup plus de temps pour libérer la salle d’opération à la suite des cas assistés par robotique (14 c. 11 min, p = 0,015). Nous avons aussi observé que l’anesthésie tend à être plus longue en moyenne dans le cas des interventions assistées par robotique (23 c. 15 min). En ce qui concerne la courbe d’apprentissage, il a fallu en moyenne plus de temps opératoire pour pratiquer les 3 premières interventions assistées par robotique que les 3 dernières (101 c. 80 min), mais cette différence n’était pas significative. Depuis cette expérience, l’équipe a pratiqué avec confiance des interventions biliaires, pancréatiques, gastro-œsophagiques, intestinales et colorectales assistées par robotique.
Conclusion
Il est possible de pratiquer en toute confiance la cholécystectomie assistée par robotique, mais étant donné l’augmentation importante des ressources utilisées en salle d’opération, on ne peut la justifier pour utilisation de routine. Notre expérience démontre toutefois que la cholécystectomie assistée par robotique est un moyen pour les chirurgiens généraux d’acquérir de la confiance dans l’exécution d’interventions avancées assistées par robotique.
Specialties like urology and cardiac surgery have seen an explosion in the use of da Vinci robotics to augment minimally invasive approaches. The likely rationale for this is the added dexterity provided by Intuitive Surgical’s Endowrist technology, which improves ease in performing complex tasks such as suturing. Unlike other specialties, there are few, but growing, fellowship opportunities for general surgeons in advanced robotics. Despite this, the rapid proliferation of surgical robotics has led to several reports of successful general surgical operations completed with robot assistance.1–5 Owing to the shortage of formal training opportunities, general surgeons are forced to incorporate the use of advanced robotics into their existing practices.
In general surgery, advanced robotics will likely find its place in the most complex laparoscopic procedures where the enhanced dexterity and superior visualization will extend the feasibility of the minimally invasive approach, particularly in patients requiring advanced suturing and precise tissue dissection. If that is the case, how then can surgeons initiate robotics into their practices? We hypothesized that robot-assisted laparoscopic cholecystectomy would be a safe and effective bridge to advanced robotics in general surgery.
Laparoscopic cholecystectomy is a prime operation with which to begin robot applications for several reasons. First, gallstone disease is the most common of all abdominal diseases for which patients are admitted to hospital in developed countries,6 making it the most commonly performed laparoscopic procedure.7 Moreover, it is an operation with a very standardized approach to prevent complications. This standardized approach has an added advantage of having aspects that may be more broadly applied to other more complex minimally invasive operations. For example, the dissection of the Calot triangle is analogous to dissection and isolation of vasculature, the cystic duct and artery can be tied instead of using clips, and removal of the gallbladder from the gallbladder fossa requires avascular dissection and the appreciation of tissue planes. Therefore, robotic cholecystectomy may allow general surgeons to acquire clinical da Vinci experience in a familiar setting.
Methods
To begin robotic operations, 2 general surgeons (C.M.S., W.D.) enrolled in the Intuitive Surgical da Vinci training course. Both surgeons had advanced laparoscopic surgical skills prior to robotic training. Both surgeons agreed to work together on all robot-assisted procedures. It was agreed that the primary surgeon could move on to more advanced operations only after performing a minimum of 10 da Vinci–assisted laparoscopic cholecystectomies.
We included all patients referred to the outpatient general surgery clinic for consideration for elective cholecystectomy as potential candidates for a da Vinci–assisted approach. We deemed patients unsuitable if they had contraindications to laparoscopic cholecystectomy, if they could not provide their own consent and if they had a history of extensive upper abdominal surgery. Not all patients clearing even these criteria were offered the da Vinci approach, as the ultimate choice for inclusion was left to the surgeons’ discretion.
If patients had reasonable indication for cholecystectomy, we obtained consent for a novel da Vinci–assisted approach for their surgeries. We informed patients of the innovative nature of this approach and the surgeons’ experience using this technology. No proven benefit to performing the surgery with this technology was offered. We explained that we were using this familiar operation as a means to evaluate and improve skills with the da Vinci surgical system with the intent of moving on to more advanced procedures.
The primary outcome of this retrospective series was safety as indicated by complications, transition to laparoscopy and conversion to open surgery. The secondary outcome was learning curve, as indicated by duration of surgery. We compared these outcomes in a nonrandomized fashion with a cohort of contemporaneous laparoscopic cholecystectomy controls.
Robotic procedure
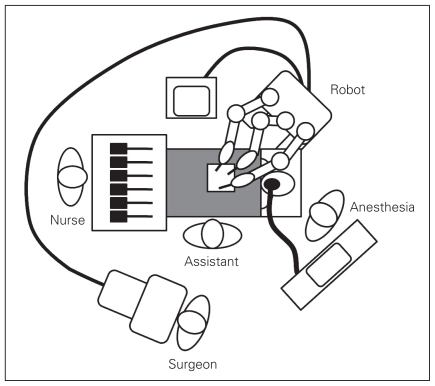
We prepared patients in both groups in the same way: with parenteral cefazolin but no antithrombosis prophylaxis unless indicated by age or comorbidity. Patients received general anesthesia. Using open first trocar entry, we inserted a balloon-tip 12-mm trocar at the umbilicus. We obtained a pneumoperitoneum of 14 mm Hg. Then we inserted 2 8-mm da Vinci trocars under direct visualization in the right lower and left upper quadrants to triangulate the gallbladder, and we placed a 5-mm accessory port in the right upper quadrant. We then docked da Vinci by bringing it over the patients’ right shoulders1 (Fig. 1). Using an accessory port in the right upper quadrant for cephalad traction of the fundus of the gallbladder, dissection of the Calot triangle commenced such that we obtained the critical view. Once the critical view was obtained, we then tied off the cystic duct and artery using 0-silk ties proximally and distally. They were then divided, and we resected the gallbladder from the gallbladder fossa using cautery. We placed the specimen in an Endocatch bag and removed it through the umbilical port. We then closed the port sites and skin.
Fig. 1.
Arrangement of the operating room for da Vinci cholecystectomy.
Statistical analysis
We anticipated that event rates of the primary outcome of safety would be low, so we descriptively compared this outcome with complications in the control arm of patients receiving contemporaneous laparoscopic surgery. We compared the mean duration of surgery in both groups using the Student t test. To further evaluate the presence of a learning curve with robotic cholecystectomy, we compared the mean duration of surgery for the first 3 patients in the robotic arm with that of the last 3 patients in the robotic arm using the Student t test.
Results
A total of 16 patients underwent robotic cholecystectomy. These patients were compared with 20 controls who received contemporaneous laparoscopic cholecystectomy (Table 1). Indications for surgery were most commonly biliary colic and chronic cholecystitis (Table 2). Regarding the primary outcome of safety, there were no serious complications in either group. Two patients in the robotic group experienced complications: 1 experienced an incisional hernia at an 8-mm port site requiring elective repair, 1 had a retained biliary stone that passed with no further treatment. There were no transitions to laparoscopy, nor were there any conversions to open surgery. The only complication in the laparoscopic group was that 1 patient required hospital admission for slow awakening from anesthetic.
Table 1.
Comparison of laparoscopic and robotic surgery patients
| Type of surgery |
||
|---|---|---|
| Characteristic | Laparoscopic | Robotic |
| No. of patients | 20 | 16 |
| Mean age, yr | 53.7 | 48.9 |
| Proportion female, no. (%) | 14 (70) | 9 (56) |
| Comorbidities, no. | 3 obese | 1 smoker |
| 3 chronic lung disease | 1 coronary artery disease | |
| 1 asthma | 1 diabetes | |
| 2 coronary artery disease | ||
| 1 stroke | ||
| 3 smokers | ||
| 2 diabetes | ||
| Previous abdominal surgery, no. | 2 | 1 |
Table 2.
Indications for surgery
| Diagnosis | Type of surgery, no. | |
|---|---|---|
| Laparoscopic | Robotic | |
| Biliary colic | 12 | 9 |
| Chronic cholecystitis | 4 | 5 |
| Biliary dyskinesia | 2 | — |
| Previous choledocholithiasis | 2 | 2 |
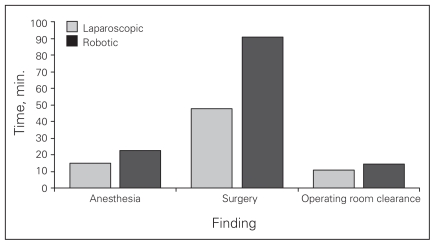
Figure 2 summarizes our key findings. The amount of surgical time required for robotic procedures, including docking, was significantly greater than that in laparoscopic procedures (91 v. 48 min, p < 0.001). Similarly, the time needed to clear the operating room after the completion of the procedure was significantly longer in the robotic group (14 v. 11 min, p = 0.015). Anesthesia time was also longer in the robotic group (23 v. 15 min), though this was not a significant finding.
Fig. 2.
Robotic cholecystectomy takes more time.
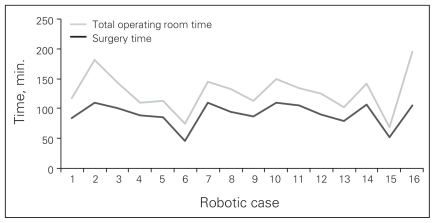
Regarding learning curve, the first 3 robotic procedures took longer to perform than the last 3 (101 v. 80 min), though this finding was not statistically significant. Figure 3 demonstrates the total duration of surgery for all of the patients in the robotic arm. There are 3 distinct peaks and troughs in the learning curve that correspond to the surgeons increasing the presumed level of difficulty of the procedures as they became more accustomed to the robotic approach.
Fig. 3.
Learning curve for robotic cholecystectomy.
It was not possible to track preoperative operating room set-up time as the robotic procedures were the first of the day at our institution. A group of dedicated nurses came early the day of surgery to set-up the operating room for da Vinci. Alternatively, the robot and the room were prepared the preceding night.
Discussion
The primary outcome of our series was safety, as indicated by complications. The overall rate of all complications as well as retained stones in our series is in keeping with historic controls in the literature.8 After experiencing an 8-mm port-site hernia, we started routinely suturing closed the fascia at 8-mm ports in all patients. There are other published reports of 8-mm port-site hernias;9 as such, it should be broadly recommended that any port greater than 5 mm in diameter should be formally sutured closed.
Although robotic procedures took substantially more time than laparoscopic ones in our experience, our total duration of surgery is in keeping with that in other centres implementing da Vinci (Table 3). As our surgical and nursing teams become more accustomed to da Vinci, we see a trend toward decreased duration of surgery. Our results show that the work times for surgeons, anesthesiologists and nurses are longer for robotic procedures. This suggests that the entire operating room team experiences a learning curve with the robot. The anesthesiologist no longer has access to the patient’s head during da Vinci operations owing to the bulk of the robot; perhaps that influences the time it takes to prepare the patient for surgery. Likewise, despite a formal training program through Intuitive Surgical, the nurses are not necessarily familiar with all robotic instruments. Similarly, the size of the robot limits the work space in the operating theatre, making the work of the circulating staff more challenging. These challenges should be overcome with experience; however, in the short-term, we cannot justify all patients being offered robotic cholecystectomy owing to the need to move patients through the system in a timely manner.
Table 3.
Mean duration of surgery in published series of da Vinci cholecystectomy
| Study | No. of patients | Mean duration of surgery, min |
|---|---|---|
| Present study | 16 | 91 |
| Vidovszky et al.10 | 48 | 77 |
| Heemskerk et al.11 | 12 | 150 |
| Giulianotti et al.1 | 66 | 85 |
| Perez et al.12 | 20 | 152 |
| Ruurda et al.13 | 35 | 97* |
| Kim et al.14 | 10 | 57 |
| Talamini et al.15 | 8 | 167 |
| Hashizume et al.16 | 6 | 118 |
| Cadière et al.17 | 48 | 70 |
| Chitwood et al.18 | 20 | 62 |
| Hanisch et al.19 | 5 | 95 |
Indicated time is the sum of the median set-up and median surgical times.
There are only a handful of centres that have a diverse and varied experience with advanced robotics. As we have, these centres all began with routine procedures like cholecystectomy and fundoplication before advancing to more complex ones such as pancreaticoduodenectomy or liver resection.1,20 It would appear that the opportunity to learn robotics on a routine, everyday procedure that mimics more complex ones is unique to general surgery.
Our experience raises other potential research questions. What is an appropriate number of procedures to perform before moving on to more advanced operations? Does experience with robotic cholecystectomy actually result in better performance of more advanced operations? More research should be dedicated to answering these questions.
To our knowledge, ours is the first reported series of da Vinci–assisted gallbladder surgery in the Canadian health care environment. We have demonstrated that robotic cholecystectomy can be performed safely. Although cholecystectomy appears to be a safe, standardized procedure on which to begin robotic surgery, the advantages of robotics will likely be realized with the most complicated abdominal operations. As a result of our experience, we have gone on to confidently perform more advanced robotic procedures, including common bile duct exploration,21 antireflux surgery, gastroesophageal surgery, pancreatic surgery, colorectal operations and a hand-sewn intestinal anastomosis.
The advantages of the da Vinci system are the dexterity of the Endowrist, allowing complex minimally invasive tasks as well as 3-dimensional visualization of the surgical field. We found that both of these advantages far outweighed the absence of tactile feedback. The improved visualization and dexterity made the dissection of the Calot triangle facile; however, we cannot conclude that the robot was more effective than standard laparoscopic instruments at this time. Subjectively, we did not find that da Vinci facilitates dissection of an inflamed gallbladder any better than laparoscopy, mainly because the complete absence of haptics seems to override the gains in dexterity and visualization.
There are other limitations to the robotic system other than the absence of tactile feedback. From a staffing perspective, robotic surgery requires the presence of a second experienced surgeon at the bedside to exchange the robotic instruments, retract for exposure and assist with the procedure.
Even though robotic cholecystectomy can be performed reliably and is a useful tool for skill acquisition, it cannot be justified for routine use owing to the increase in operating room resources.
Conclusion
Our experience demonstrates that robotic cholecystectomy can be performed reliably. However, owing to the significant increase in operating room resources, it cannot be justified for routine use. Robotic cholecystectomy does provide the opportunity to develop familiarity with a wide set of procedure components that are ultimately applicable to more advanced procedures. As such, it is one means by which general surgeons may gain confidence in advanced robotics.
Footnotes
This paper was a podium presentation at the Minimally Invasive and Robotic Association (MIRA) 3rd International Congress, Rome, Italy, Jan. 24–26, 2008
Competing interests: None declared.
Contributors: All authors contributed to study design, reviewed the article and approved publication. Drs. Jayaraman and Schlachta acquired and analyzed the data and wrote the article.
References
- 1.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–84. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 2.Marescaux J, Smith MK, Folscher D, et al. Telerobotic laparoscopic cholecystectomy: initial clinical experience with 25 patients. Ann Surg. 2001;234:1–7. doi: 10.1097/00000658-200107000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadière GB, Himpens J, Vertruyen M, et al. Nissen fundoplication done by remotely controlled robotic technique. Ann Chir. 1999;53:137–41. [PubMed] [Google Scholar]
- 4.Cadière GB, Himpens J, Vertruyen M, et al. The world’s first obesity surgery performed by a surgeon at a distance. Obes Surg. 1999;9:206–9. doi: 10.1381/096089299765553539. [DOI] [PubMed] [Google Scholar]
- 5.Chapman WH, III, Albrecht RJ, Kim VB, et al. Computer-assisted laparoscopic splenectomy with the da Vinci trade mark surgical robot. J Laparoendosc Adv Surg Tech A. 2002;12:155–9. doi: 10.1089/10926420260188038. [DOI] [PubMed] [Google Scholar]
- 6.Rosenmüller M, Haapamäki MM, Nordin P, et al. Cholecystectomy in Sweden 2000–2003: a nationwide study on procedures, patient characteristics, and mortality. BMC Gastroenterol. 2007;17(7):35. doi: 10.1186/1471-230X-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varadarajulu S, Tamhane A, Drelichman ER. Patient perception of natural orifice transluminal endoscopic surgery as a technique for cholecystectomy. Gastrointest Endosc. 2008;67:854–60. doi: 10.1016/j.gie.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 8.Keus F, de Jong JAF, Gooszen HG, et al. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;(4):CD006231. doi: 10.1002/14651858.CD006231. [DOI] [PubMed] [Google Scholar]
- 9.Tonouchi H, Ohmori Y, Kobayashi M, et al. Trocar site hernia. Arch Surg. 2004;139:1248–56. doi: 10.1001/archsurg.139.11.1248. [DOI] [PubMed] [Google Scholar]
- 10.Vidovszky TJ, Smith W, Ghosh J, et al. Robotic cholecystectomy: learning curve, advantages, and limitations. J Surg Res. 2006;136:172–8. doi: 10.1016/j.jss.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Heemskerk J, van Dam R, van Gamert WG, et al. First results after introduction of the four-armed da Vinci Surgical System in fully robotic laparoscopic cholecystectomy. Dig Surg. 2005;22:426–31. doi: 10.1159/000091445. [DOI] [PubMed] [Google Scholar]
- 12.Perez A, Zinner MJ, Ashley SW, et al. What is the value of telerobotic technology in gastrointestinal surgery. Surg Endosc. 2003;17:811–3. doi: 10.1007/s00464-002-8561-z. [DOI] [PubMed] [Google Scholar]
- 13.Ruurda JP, Broeders IA, Simmermacher RP, et al. Feasibility of robot-assisted laparoscopic surgery: an evaluation of 35 robot-assisted laparoscopic cholecystectomies. Surg Laparosc Endosc Percutan Tech. 2002;12:41–5. doi: 10.1097/00129689-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Kim VB, Chapman WH, Albrecht RJ, et al. Early experience with telemanipulative robot-assisted laparoscopic cholecystectomy using da Vinci. Surg Laparosc Endosc Percutan Tech. 2002;12:33–40. doi: 10.1097/00129689-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Talamini M, Campbell K, Stanfield C. Robotic gastrointestinal surgery: early experience and system description. J Laparoendosc Adv Surg Tech A. 2002;12:225–32. doi: 10.1089/109264202760267970. [DOI] [PubMed] [Google Scholar]
- 16.Hashizume M, Shimada A, Tomikawa M, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. 2002;16:1187–91. doi: 10.1007/s004640080154. [DOI] [PubMed] [Google Scholar]
- 17.Cadière GB, Himpens J, Germay O, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg. 2001;25:1467–77. doi: 10.1007/s00268-001-0132-2. [DOI] [PubMed] [Google Scholar]
- 18.Chitwood WR, Jr, Nifong LW, Chapman WH, et al. Robotic surgical training in an academic institution. Ann Surg. 2001;234:475–84. doi: 10.1097/00000658-200110000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanisch E, Markus B, Gutt C, et al. Robot-assisted laparoscopic cholecystectomy and fundoplication — initial experiences with the da Vinci system. Chirurg. 2001;72:286–8. doi: 10.1007/s001040051307. [DOI] [PubMed] [Google Scholar]
- 20.Ruurda JP, Draaisma WA, van Hillegersberg R. Robot-assisted endoscopic surgery: a four-year single-center experience. Dig Surg. 2005;22:313–20. doi: 10.1159/000088628. [DOI] [PubMed] [Google Scholar]
- 21.Jayaraman S, Davies W, Schlachta CM. Robot-assisted minimally invasive common bile duct exploration: a Canadian first. Can J Surg. 2008;51:E93–4. [PMC free article] [PubMed] [Google Scholar]