Abstract
The basolateral complex (BLA) and central nucleus (CEA) of the amygdala play critical roles in associative learning, including Pavlovian conditioning. However, the precise role for these structures in Pavlovian conditioning is not clear. Recent work in appetitive conditioning paradigms suggests that the amygdala, particularly the BLA, has an important role in representing the value of the unconditioned stimulus (US). It is not known whether the amygdala performs such a function in aversive paradigms, such as Pavlovian fear conditioning in rats. To address this issue, Experiments 1 and 2 used temporary pharmacological inactivation of the amygdala prior to a US inflation procedure to assess its role in revaluing shock USs after either overtraining (Experiment 1) or limited training (Experiment 2), respectively. Inactivation of the BLA or CEA during the inflation session did not affect subsequent increases in conditioned freezing observed to either the tone conditioned stimulus (CS) or the conditioning context in either experiment. In Experiment 3, NBQX infusions into the BLA impaired the acquisition of auditory fear conditioning with an inflation-magnitude US, indicating that the amygdala is required for associative learning with intense USs. Together, these results suggest that the amygdala is not required for revaluing an aversive US despite being required for the acquisition of fear to that US.
Pavlovian fear conditioning in rats is a behavioral model used to investigate the neurobiology underlying the development and maintenance of fear learning and memory (Grillon et al. 1996; LeDoux 1998, 2000; Bouton et al. 2001; Maren 2001b, 2005; Kim and Jung 2006). In this model, an innocuous conditioned stimulus (CS), such as a tone, is paired with an aversive unconditioned stimulus (US), such as a footshock. After one or more pairings, the rat learns that the CS predicts the US. As a consequence, CS presentations alone elicit a conditioned fear response (CR), which includes increases in heart rate, arterial blood pressure, hypoalgesia, potentiated acoustic startle, stress hormone release, and freezing (somatomotor immobility).
The amygdala has been identified as one of the major regions in which fear memories are encoded and stored. Within the amygdala, the basolateral complex of the amygdala (BLA; consisting of the lateral, basolateral, and basomedial nuclei) and the central nucleus of the amygdala (CEA) receive convergent CS and US information and are involved in the acquisition of fear memories (LeDoux 1998, 2000; Fendt and Fanselow 1999; Davis and Whalen 2001; Maren 2001b; Schafe et al. 2001; Fanselow and Gale 2003; Wilensky et al. 2006; Zimmerman et al. 2007). In addition, the CEA has an important role in the expression of fear CRs (Fendt and Fanselow 1999; LeDoux 2000; Davis and Whalen 2001; Maren 2001b; Fanselow and Gale 2003). In support of this, many studies have shown that either permanent or temporary lesions of the BLA or CEA prevent the acquisition and/or expression of fear memories (Helmstetter 1992; Helmstetter and Bellgowan 1994; Campeau and Davis 1995; Maren et al. 1996a,b; Killcross et al. 1997; Muller et al. 1997; Walker and Davis 1997; Cousens and Otto 1998; Maren 1998, 1999, 2001a,b; Wilensky et al. 1999, 2000, 2006; Goosens and Maren 2001, 2003; Nader et al. 2001; Fanselow and Gale 2003; Gale et al. 2004; Koo et al. 2004; Zimmerman et al. 2007).
In addition to its role in encoding CS–US associations during conditioning, recent work suggests that the amygdala is also involved in representing properties of the US itself. For example, temporary or permanent lesions of the BLA reduce both decrements in conditioned responding after devaluation of a food US (Hatfield et al. 1996; Killcross et al. 1997; Blundell et al. 2001; Balleine et al. 2003; Everitt et al. 2003; Pickens et al. 2003; Holland 2004) and increments in conditional responding after inflation of a shock US (Fanselow and Gale 2003). Moreover, recent electrophysiological studies in primates indicate that amygdala neurons represent the value of both aversive and appetitive outcomes (Paton et al. 2006; Belova et al. 2007, 2008; Salzman et al. 2007). These studies suggest that one function of the BLA is to represent specific properties of biologically significant events, such as the food or shock USs that are typically used in Pavlovian conditioning paradigms. By this view, the BLA may represent specific sensory properties of USs that shape the nature of learned behavioral responses to the US (Balleine and Killcross 2006) and allow CSs to gain access to the incentive value of the US (Everitt et al. 2003).
In contrast to this view, we recently reported that rats with neurotoxic BLA lesions exhibit normal US revaluation after Pavlovian fear conditioning (Rabinak and Maren 2008). In this study, auditory fear conditioning (75 CS–US trials) with a moderate footshock (1 mA) was followed by several exposures (five US-alone trials) to an intense footshock (3 mA) during an inflation session. Both intact rats and rats with BLA lesions exhibit a robust increase in conditional freezing to the auditory CS during a subsequent retention test (Rabinak and Maren 2008). Control experiments suggested that this was due to a revaluation of the US with which the CS was associated, rather than nonassociative sensitization of fear engendered by exposure to intense shock. These data reveal that the BLA may not be necessary for representing properties of shock USs during Pavlovian fear conditioning. To address these issues further, we have examined the consequence of reversible pharmacological manipulations of the amygdala during US inflation on conditional fear responses established with either extensive or limited training.
Results
Experiment 1: Inactivation of the BLA during US inflation does not impair US revaluation of overtrained fear memories
A recent study from our laboratory revealed that rats with neurotoxic BLA lesions exhibit normal inflation of fear when a series of intense USs was delivered after overtraining (Rabinak and Maren 2008). Because lesions allow for recovery of function and compensation by alternate neural circuits, in the present experiment we examined the contribution of the amygdala to US revaluation using temporary brain lesions. To this end, we reversibly inactivated the BLA with NBQX, an antagonist of the AMPA-subtype of glutamate receptors, during the inflation procedure to examine whether the BLA is required to encode changes in the value of an aversive US. Prior to US inflation, auditory fear conditioning was established using an overtraining procedure to directly compare the results with those obtained with neurotoxic BLA lesions (Rabinak and Maren 2008).
Histology
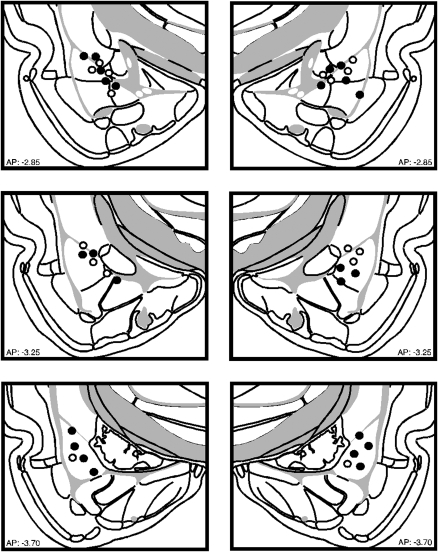
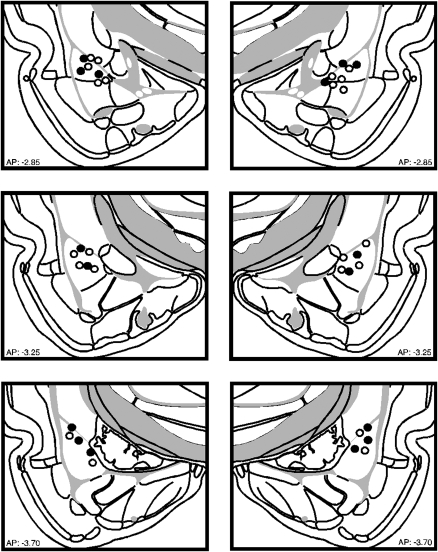
Figure 1 illustrates the cannula implants of rats included in the statistical analysis for Experiment 1. Nine rats were excluded from the analyses because their cannulae placements missed the BLA. This yielded the following groups designated by drug type during inflation (NBQX; vehicle, VEH) and inflation condition (inflation, INF; no inflation, NoINF): NBQX-INF (n = 6), NBQX-NoINF (n = 6), VEH-INF (n = 6), and VEH-NoINF (n = 5).
Figure 1.
Schematic representation of the locations of included cannula placements for the infusion of NBQX (●) or VEH (○) in the BLA for Experiment 1. (Coronal brain images adapted from Swanson [2004] and reprinted with permission from Academic Press ©2004.)
Behavior
Freezing behavior during each behavioral session of Experiment 1 is shown in Figure 2. Although each experimental group is represented in each panel of the figure, it is important to note that both the drug infusion and inflation manipulation occurred only during the “Inflate” session. Hence, Figure 2A indicates that all groups acquired comparable levels of post-shock freezing during the training session. This observation was confirmed by a significant main effect of time (15 trial blocks) (F(1,19) = 188.3, P < 0.0001) in the ANOVA.
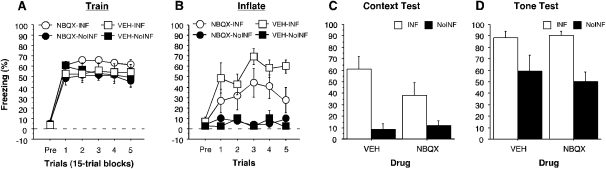
Figure 2.
Conditioned freezing during the overtraining session, inflation session, context, and tone tests (left to right, respectively) (Experiment 1). (A) Mean percentage of freezing (± SEM) during the 75-min overtraining session (15-trial blocks). (B) Mean percentage of freezing (± SEM) during the five-trial inflation session. For both line graphs, groups are denoted as follows: NBQX-INF group (○), VEH-INF group (□), NBQX-NoINF group (●), VEH-NoINF group (■). (C) Mean percentage of freezing (± SEM) across the 10-min context test. (D) Mean percentage of freezing (± SEM) during the two-trial tone test. Data are an average of freezing during the ITI periods. In both bar graphs, data are shown for INF groups (white bar) and the NoINF groups (black bar) within each drug type.
Twenty-four hours after fear conditioning, rats were transported to a novel context for the inflation session (Fig. 2B). It is apparent that rats receiving intense inflation shocks (INF) displayed greater levels of freezing than the no-inflation (NoINF) controls, which did not receive footshock. This observation was confirmed by a two-way ANOVA with variables of drug (VEH or NBQX) and inflation (INF or NoINF) on post-shock freezing behavior averaged across the inflation session (main effect of inflation, F(1,19) = 34.9; P < 0.0001). Although there was no significant main effect of drug (F(1,19) = 2.3, P = 0.15) or interaction between drug and inflation condition during the inflation session (F(1,19) = 3.2, P = 0.09), there was nonetheless a trend for NBQX infusion in the BLA to reduce conditioned freezing to the inflation shocks. Indeed, pairwise comparisons of the group means (P < 0.05) revealed that rats in the VEH-INF group exhibited significantly greater levels of freezing than rats in the NBQX-INF condition. Importantly, rats infused with NBQX in the BLA exhibited comparable shock-induced activity bursts during the inflation session (F(1,10) = 1.5; P = 0.25; data not shown).
The influence of US inflation on conditional fear was assessed in separate retention tests for both the conditioning context (Fig. 2C) and the tone CS (Fig. 2D). As shown in Figure 2, C and D, rats in both the VEH-INF and NBQX-INF groups exhibited greater levels of freezing behavior than non-inflated controls. These observations were confirmed by two-way ANOVAs that revealed significant main effects of inflation condition (context: F(1,19) = 20.0; P = 0.0003; tone: F(1,42) = 18.0, P = 0.0001), without significant effects of drug (context: F(1,19) = 1.2, P = 0.29; tone: F(1,42) = 0.2, P = 0.66) or drug × inflation interactions (context: F(1,19) = 2.1, P = 0.16; tone: F(1,42) = 0.5, P = 0.48). Both inflation groups (NBQX-INF and VEH-INF) displayed significantly higher levels of freezing when compared with the non-inflated groups (NBQX-NoINF and VEH-NoINF; P < 0.05 for all comparisons). Within the non-inflated groups, freezing to the context tended to be lower than freezing to the tone, suggesting that the tone CS overshadowed the context (Rescorla and Wagner 1972). Prior to the first tone presentation on the test, freezing was low and did not differ across the groups (F(3,19) = 2.4; P = 0.10; mean percent freezing = VEH-INF, 29.9% ± 11.9%; VEH-NoINF, 1.8% ± 1.7%; NBQX-INF, 14.7% ± 4.0%; NBQX-NoINF, 12.0% ± 6.2%). These data indicate that AMPA receptor antagonism in the BLA during exposure to intense shock USs does not prevent the inflation of fear to an auditory CS that had been paired with that US.
Experiment 2: Inactivation of the BLA or CEA during US inflation does not impair US revaluation of weakly trained fear memories
Consistent with our earlier report with BLA lesions, Experiment 1 reveals that reversible inactivation of the BLA does not disrupt inflation of overtrained fear memories. However, overtrained fear memories may have been resilient to manipulations of the amygdala as a consequence of the overtraining procedure. For instance, Nader and colleagues find that, unlike weak memories, overtrained memories are immune to disruption by post-reactivation infusions of protein synthesis inhibitors in the BLA (Wang et al. 2009). To address these issues, Experiment 2 examined the consequence of NBQX infusions in either the BLA or CEA on the inflation of fear memories acquired after limited training (five CS–US trials). Although the CEA has not been previously implicated in inflation, recent data suggest that it has a broader role in aversive learning processes than previously suggested (Maren 1999; Wilensky et al. 2006; Zimmerman et al. 2007) and may therefore play a role in US revaluation.
Histology
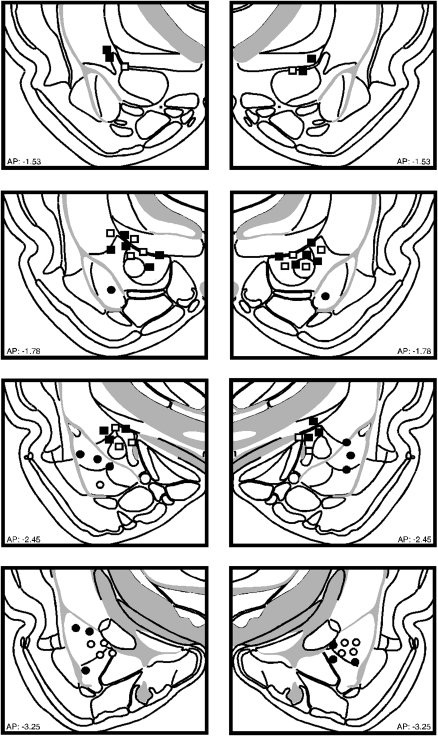
Figure 3 illustrates the cannula implants of rats included in the statistical analysis for Experiment 2. Six rats were excluded from analyses because the cannulae placements missed the BLA or CEA. Rats receiving vehicle infusions into the BLA or CEA did not differ at any point in the experiment and were collapsed for the statistical analysis. This yielded the following groups designated by drug and inflation condition (inflation, INF; no inflation, NoINF): VEH-INF (n = 14), and VEH-NoINF (n = 12), BLA-INF (n = 8), BLA-NoINF (n = 8), CEA-INF (n = 8), CEA-NoINF (n = 8).
Figure 3.
Schematic representation of the locations of included cannula placements for the infusion of NBQX (closed) or VEH (open) in the BLA (circles) or CEA (squares) for Experiment 2. (Coronal brain images adapted from Swanson [2004] and reprinted with permission from Academic Press ©2004.)
Behavior
Post-shock freezing during the conditioning session in Experiment 2 is shown in Figure 4A. As in Experiment 1, the group assignments refer to drug and inflation manipulations conducted in the subsequent inflation session. All groups exhibited a similar increase in post-shock freezing across the session (trial: F(5,180) = 57.9, P < 0.0001), and did not differ in their levels of freezing.
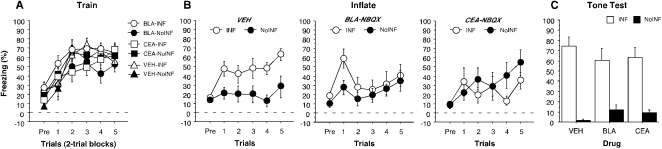
Figure 4.
Conditioned freezing during the training session, inflation session, and tone test (left to right, respectively) (Experiment 2). (A) Mean percentage of freezing (± SEM) during the 10-trial (two-trial blocks) training session is shown for BLA-INF (○), CEA-INF (□), VEH-INF (△), BLA-NoINF (●), CEA-NoINF (■), and VEH-NoINF (▲). Conditioned freezing during the inflation session (Experiment 2). (B) Mean percentage of freezing (± SEM) during the five-trial inflation session for INF (○) and NoINF (●) for rats that received vehicle infusions (far left graph; collapsed across brain areas), NBQX infused into the BLA (middle graph), or NBQX infused into the CEA (right graph). Data are an average of freezing during the ITI periods. (C) Mean percentage of freezing (± SEM) during the two-trial tone test for the INF groups (white bars) and for the NoINF groups (black bar) within each drug group.
Twenty-four hours after fear conditioning, rats were transported to a novel context for the inflation session (Fig. 4B). It is apparent from these data that NBQX infusions into the amygdala, particularly the CEA, reduced freezing during the inflation session. A two-way ANOVA performed on the average post-shock freezing across the inflation session revealed a trend toward a significant interaction between drug and inflation (F(2,36) = 3.1, P = 0.058). Post hoc comparisons revealed that while rats in the VEH-INF group exhibited greater freezing relative to their no-inflation controls (VEH-NoINF), rats receiving NBQX in either the BLA or CEA exhibited impaired inflation. Hence, AMPA receptor antagonism in the amygdala reduced post-shock freezing to intense shocks during the inflation session. Although NBQX infused into the amygdala reduced conditioned freezing to the inflation shocks, it did not affect shock reactivity (F(2,20) = 1.6, P = 0.22; data not shown).
Forty-eight hours after conditioning, the rats were placed in a novel context to assess conditional freezing to the auditory CS (Fig. 4C). This experimental design was developed from a previous experimental protocol that did not include a context retention test; therefore, the context retention test was omitted from this experiment. It is apparent from these data that all of the groups receiving inflation shocks exhibited greater levels of freezing than their non-inflated controls and that NBQX infusion into the amygdala did not reduce this effect. These observations were confirmed by a two-way ANOVA that revealed a significant main effect of inflation condition (F(1,35) = 74.8, P < 0.0001), without a significant effect of drug type (F(2,35) = 0.03, P = 0.97) or an interaction between drug and inflation (F(2,35) = 1.2; P = 0.3). Prior to the first tone presentation, freezing was low in all groups, and it significantly increased after the first tone presentation (F(1,35) = 66.3, P < 0.0001) even though rats that received inflated shocks froze higher than rats that did not undergo inflation procedures prior to the first tone presentation (F(1,35) = 19.1, P < 0.0001; mean percent freezing = VEH-INF, 22.3% ± 6.7%; VEH-NoINF, 0.4% ± 0.2%; BLA-INF, 19.1% ± 5.6%; BLA-NoINF, 2.5% ± 1.3%; CEA-INF, 24.6% ± 10.4%; CEA-NoINF, 1.1% ± 0.5%). During the tone test the non-inflated groups displayed low levels of freezing, which may be due to generalized extinction from exposure to similar chambers during the inflation session. Overall, similar to Experiment 1, these data reveal that AMPA receptor antagonism in the BLA or CEA reduces freezing to intense shocks during the inflation session, but does not prevent revaluation of a weakly trained CS–US memory.
Experiment 3: Inactivation of the BLA impairs auditory fear conditioning to an intense US
The previous studies suggest that the amygdala is not required for inflation of an aversive US. However, it is possible that the amygdala is not involved in processing intense USs, in general. To assess this possibility, we examined whether BLA infusions of NBQX would impair the acquisition of fear conditioning with an intense, inflation-magnitude US.
Histology
Figure 5 illustrates the cannula implants of rats included in the statistical analysis for Experiment 3. Two rats were excluded from analyses because the cannulae placements missed the BLA. This yielded the following groups designated by drug: VEH (n = 6) and NBQX (n = 10).
Figure 5.
Schematic representation of the locations of included cannula placements for the infusion of NBQX (●) or VEH (○) in the BLA for Experiment 3. (Coronal brain images adapted from Swanson [2004] and reprinted with permission from Academic Press ©2004.)
Behavior
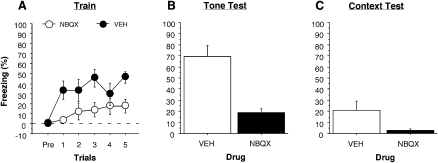
Post-shock freezing during the conditioning session is shown in Figure 6A. It is apparent from these data that NBQX infusions into the BLA reduced freezing during the conditioning session. A two-way ANOVA performed on the average post-shock freezing across the conditioning session revealed a significant main effect of drug, (F(1,14) = 6.8, P < 0.021).
Figure 6.
Conditioned freezing during the training session, tone, and context tests (left to right, respectively) (Experiment 3). (A) Mean percentage of freezing (± SEM) during the 5-min training session. Groups are denoted as follows: NBQX (○),VEH (●). (B) Mean percentage of freezing (± SEM) during the 10-trial tone test. (C) Mean percentage of freezing (± SEM) across the 10-min context test. Data are an average of freezing during the ITI periods. In both bar graphs, data are shown for NBQX (white bar) and the VEH group (black bar).
Twenty-four hours after fear conditioning, rats were transported to a novel context (Context B) for a context exposure session. This session was included to reduce any generalized contextual fear that may have transferred from the conditioning context (Context A) to the tone test context (Context B), and to equate the procedure with the inflation experiments. During the exposure session, both groups (VEH and NBQX) displayed low-levels of freezing (F(1,14) = 2.9, P = 0.1066) (data not shown).
Conditional fear was assessed in separate retention tests for both the tone CS (Fig. 6B) and the conditioning context (Fig. 6C) conducted 24 and 48 h after context exposure, respectively. As shown in Figure 6, B and C, rats in the VEH group exhibited more freezing than the NBQX group. This observation was confirmed by a two-way ANOVA that revealed a significant main effect of drug (tone: F(1,14) = 32.2, P < 0.0001; context: F(1,14) = 7.9, P < 0.01). These results indicate that NBQX impaired fear conditioning acquired with high-intensity footshocks. Therefore, the failure to disrupt inflation with NBQX in the previous experiments (Experiments 1 and 2) was not because it was ineffective at blocking associative learning processes in the amygdala.
Discussion
The present experiments used temporary pharmacological inactivation (AMPA receptor blockade with NBQX) within the amygdala during a post-conditioning manipulation of US value (an inflation procedure) to assess the contribution of the CEA and BLA to encoding and maintaining US value. We found in two experiments (Experiments 1 and 2) that AMPA receptor blockade in the BLA did not impair the inflation of conditioned freezing. This suggests that the amygdala is not necessary for coding changes in US value. The present findings are in agreement with results from previous studies in our laboratory that found that rats with BLA lesions exhibit normal inflation after overtraining (Rabinak and Maren 2008).
In contrast to our results, many studies in appetitive conditioning paradigms indicate that the BLA is importantly involved in revaluing food USs (Hatfield et al. 1996; Killcross et al. 1997; Blundell et al. 2001; Balleine et al. 2003; Everitt et al. 2003; Pickens et al. 2003; Holland 2004; Holland and Gallagher 2004; Johnson et al. 2009). One reason the BLA may be important for US revaluation in these paradigms concerns the method used to revalue the US. For example, a common procedure for revaluing USs in appetitive paradigms is to devalue the food US by pairing it with lithium chloride (LiCl)-induced illness. In this case, the animal is given an opportunity to approach and voluntarily consume the food US prior to the LiCl injection. Perhaps the instrumental properties of this procedure (approach→consume→illness) recruit the BLA differently than in the revaluation procedure in which the animal does not volunteer to consume the US (as in shock inflation). Alternatively, the direction in which the US is revalued may also differentially recruit the BLA. In appetitive studies the US experiences a decrease in value (devaluation), whereas in our experiments the US experiences an increase in value (inflation). Indeed, studies of attention have revealed differential involvement of neural structures that depends on whether there are increases or decreases in attention (Holland and Gallagher 1993; Baxter et al. 1999). For example, the CEA mediates increases in attention when there is a shift from a predictive relationship between stimuli to a surprising relationship, but does not mediate decrements in attention (Holland and Gallagher 1999). In contrast, the hippocampus mediates decrements but not increases in attention (Holland and Gallagher 1993; Baxter et al. 1999).
Human neuroimaging studies have shown that US devaluation in an appetitive-conditioning paradigm alters blood flow responses in the amygdala, whereas this response is not altered after US inflation in an aversive paradigm (Gottfried et al. 2003; Gottfried and Dolan 2004). Furthermore, recent appetitive studies have found that the BLA is involved in reinforcer devaluation only when multiple reinforcers are used (Wellman et al. 2005; Ostlund and Balleine 2008; Johnson et al. 2009); BLA manipulations do not influence devaluation when single-outcome associations are learned (Pickens et al. 2003). Hence, differences in both the valence and number of USs may influence the degree to which the amygdala is engaged in US revaluation processes.
The finding that neither the BLA nor CEA is important for US revaluation in Pavlovian fear conditioning is somewhat surprising given the important role for both structures in the acquisition and expression of conditional fear responses. It is possible that our method of “inactivating” the CEA and BLA nuclei did not sufficiently limit synaptic transmission in the amygdala to prevent new learning about the US during the inflation session. Indeed, it has been shown that infusion of another AMPA receptor antagonist, CNQX, into the BLA spares the extinction of fear conditioning (another form of learning that updates information acquired during conditioning), whereas infusions of NMDA receptor antagonists (Falls et al. 1992; Laurent and Westbrook 2008; Laurent et al. 2008) or GABA agonists (Laurent and Westbrook 2008; Laurent et al. 2008) impair extinction. However, the possibility that AMPA receptor blockade in the BLA is not sufficient to prevent learning is made less likely by the outcome of Experiment 3. Here, we found that the acquisition of fear to an auditory CS paired with an inflation-magnitude US was dramatically impaired by NBQX infusions. This suggests that BLA-dependent learning, even with intense USs, remains sensitive to NBQX inactivation. Hence, our failure to impair inflation with amygdala NBQX infusions in Experiments 1 and 2 is not likely due to insufficient inactivation of the amygdala.
Although our findings indicate that amygdala AMPA receptors are not necessary for US inflation, it is still possible that local plasticity within the amygdala plays a role in consolidating or maintaining representations of revalued USs. It is generally accepted that de novo protein synthesis is required for the consolidation of long-term memories, as well as the reconsolidation of recently reactivated memory. Because the inflation session involves both new learning about the US (including its relative change in intensity from the original conditioning session and its association with a novel context) and serves as a reminder that likely reactivates conditioning memories of both the CS and US, it is reasonable to expect that protein synthesis inhibition shortly after the inflation session might impair retention of the CS, US, or both. In fact, Diaz-Mataix et al. (2008) have preliminary evidence suggesting that presentation of the US after conditioning reactivates the associated CS memory. Investigation into the role of plasticity within the amygdala in maintaining representations of revalued USs is currently underway.
Considerable work in appetitive conditioning paradigms suggests a role for the orbitofrontal cortex (OFC) in representing the value of food USs (Pickens et al. 2003, 2005; Holland and Gallagher 2004). For example, rats with OFC lesions made prior to devaluation of a food US are not sensitive to subsequent food devaluation (Pickens et al. 2003, 2005). The BLA is extensively and reciprocally connected with the OFC, and this circuitry may provide a means for CSs that have come into association with USs to retrieve the value of the US before engaging behavior. Alternatively, representations of shock USs may be encoded and maintained in brain regions that directly regulate defensive behavior to nociceptive stimuli, such as the midbrain periaqueductal gray (PAG) (Bandler et al. 2000a,b; Keay and Bandler 2001; Keay et al. 2001). Consistent with this possibility, recent neuroimaging data indicate that the human PAG is strongly activated by the expectation of imminent shock (Mobbs et al. 2007). Maintaining the value of aversive USs in the PAG may permit rapid augmentation of defensive behavior in response to both Pavlovian stimuli that signal aversive USs as well as the aversive USs themselves. Ultimately, it is increasingly apparent that multiple neural circuits regulate the associative relationships between conditional and unconditional stimuli and the motivational value those stimuli represent.
Materials and Methods
Experiment 1: Inactivation of the BLA during US inflation does not impair US revaluation of overtrained fear memories
Subjects and design
The subjects were 32 adult male Long–Evans rats (60–90 d old; 200–224 g; Blue Spruce) obtained from a commercial supplier (Harlan Sprague–Dawley). Upon arrival, all rats were individually housed in conventional Plexiglas hanging cages and kept on a 14 h light/10 h dark cycle (lights on at 7:00 a.m.) with free access to food and tap water. To acclimate the rats to the experimenter, they were handled daily (10–15 sec per rat) for 5 d following their arrival. All experimental procedures were conducted in accordance with the approved guidelines as stated by the University of Michigan Committee on Use and Care of Animals (UCUCA).
Prior to inflation, the rats were first divided into two equal groups: one group that received bilateral infusions of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; Sigma), an AMPA receptor antagonist, and a second group that received bilateral infusions of a vehicle control (VEH; ACSF; Sigma). NBQX is a short-acting drug with effects lasting up to ∼30 min, which is ideal for the duration of the inflation procedure (Gill et al. 1992; Lees 2000). Then, each drug group was further divided into two groups: one that received the US inflation procedure (INF) and a group that did not undergo US inflation (NoINF).
Behavioral apparatus
All sessions were conducted in eight identical rodent conditioning chambers (30 × 24 × 21 cm; MED Associates). The chambers were positioned inside sound-attenuating cabinets located in an isolated room. Each chamber was constructed of aluminum (two side walls) and Plexiglas (rear wall, ceiling, and hinged front door); the floor consisted of 19 stainless-steel rods, (4 mm diameter) spaced apart 1.5 cm (center to center). The grid floor was connected to a shock source and solid-state grid scrambler (MED Associates), which delivered the footshock US. Mounted on one wall of the chamber was a speaker to provide a distinct auditory CS and on the opposite wall was a 15-W house light; a fan provided background noise (65 dB).
Three distinct contexts were created by manipulating multiple visual, olfactory, and tactile cues: (1) Context A: 1% acetic acid odor in the chamber, house lights and room lights on, fans on in the cabinets, cabinet doors open, and grid floors; (2) Context B: 1% ammonium hydroxide odor in the chamber, red lights on in the room, house lights off, fans off in the cabinets, cabinet doors closed, and Plexiglas floors; (3) Context C: 70% ethanol odor in the chamber, house lights on, room lights off, fans off in the cabinets, cabinet doors open, and grid floors.
Each chamber rested on a load-cell platform, which was used to record chamber displacement in response to each rat's motor activity. The output from each load-cell was amplified to a level previously established to detect freezing responses. For each chamber, the load-cell amplifier output was digitized at 5 Hz (300 observations per minutes per rat) and acquired online using Threshold Activity software (MED Associates). Locomotor activity was quantified by the raw load-cell values (range 0–100), and freezing behavior was quantified by calculating the number of load-cell values below the freezing threshold (threshold = 10). However, to prevent the inclusion of momentary bouts of inactivity as freezing, (i.e., <1 sec) freezing was scored only after five or more contiguous observations below the freezing threshold (for details, see Maren 1998, 1999, 2001a). Freezing observations during each session were transformed into a percentage of total observations. In Experiments 1 and 2, sensitivity to the footshock US was measured by comparing the average locomotor activity over the 2-sec period prior to the first footshock presentation and the average locomotor activity during the first presentation of the footshock (2 sec).
Surgery
One week prior to training and after having been handled for 1 wk, each rat was anesthetized with an intraperitoneal (i.p.) injection of a Nembutal (sodium pentobarbital; 65 mg/kg body weight) and atropine methyl nitrate (0.4 mg/kg body weight) cocktail. Ocular lubricant was used to moisten the eyes. The scalp was shaved, cleaned with antiseptic (Betadine), and the rat was mounted in a stereotaxic apparatus (David Kopf Instruments). After the scalp was incised and retracted, the skull was positioned so that bregma and lambda were in the same horizontal plane. Small burr holes were drilled bilaterally in the skull to allow for the placement of 26-gauge guide cannulae (Plastics One) in the BLA (3.3 mm posterior to bregma, 5.0 mm lateral to the midline, and 6.5 mm ventral to the brain surface), along with holes for three small jeweler's screws. Dental acrylic was applied to the cannulae, screws, and skull surface to hold the guide cannulae in place. After surgery, 33-gauge dummy cannulae (16 mm; Plastics One) were inserted into the guide cannulae, and the rats were kept on a heating pad until they recovered from anesthesia before returning to their home cages. Dummy cannulae were replaced daily during the week of recovery.
Conditioning, inflation, and test procedure
Fear conditioning was conducted using an overtraining procedure. Rats were transported from their home cages in squads of eight and placed in the conditioning chambers (Context A). Chamber position and experimental group were counterbalanced for each squad. Rats received 75 paired presentations of a tone (10 sec, 2 kHz, 85 dB) that co-terminated with a footshock (1.0 mA, 2 sec) beginning 3 min after being placed in the chambers. There was a 60-sec intertrial interval (ITI), and the animals remained in the boxes 60 sec after the last footshock presentation. Twenty-four hours after overtraining and prior to the inflation procedure, rats were transported to the infusion room in squads of eight from their home cages in white 5-gallon buckets. Hamilton syringes (10 μL; Harvard Apparatus) were mounted in two infusion pumps (10 syringes/pump; Harvard Apparatus) and connected to 33-gauge internal cannula (1.0 mm longer than the implanted guide cannulae) with polyethylene tubing (A-M Systems). Dummy cannulae were removed from each rat, and internal cannulae were inserted into each guide cannula. Either NBQX (12 mg/mL dissolved in ACSF, pH 7.4; Sigma) or ACSF (same volume and rate) was infused bilaterally into the BLA (0.5 μL/side; 0.1 μL/min). One minute was allowed for diffusion of the drug into the target structure before the injectors were removed. Dummy cannulae were inserted into the guide cannulae once the injectors were removed, and the rats were immediately taken to the conditioning chambers for the inflation procedure. All rats were placed in another novel environment (Context C) for US inflation. The inflation session consisted of exposure to five high-intensity footshocks (3.0 mA, 2 sec) beginning 3 min after being placed in the chambers. There was a 60-sec ITI, and the animals remained in the boxes 60 sec after the last footshock. Rats in the NoINF group were placed in the chamber for the same duration as the rats in the inflation groups but did not receive footshocks. Forty-eight hours after conditioning, all rats were placed back into Context A for 10 min to assess contextual fear. Twenty-four hours after the context test, fear to the tone was tested by placing the rats into a third novel context (Context B) and presenting 30 tone alone presentations (10 sec, 2 kHz, 85 dB, 60 sec ITI) beginning 3 min after being placed into the chambers. Freezing behavior was measured throughout all experimental sessions.
Histology
After behavioral testing, rats were euthanized with an overdose of sodium pentobarbital (i.p. 100 mg/kg) and were transcardially perfused with physiological saline followed by 10% formalin. Brains were removed and post-fixed in 10% formalin followed by 10% formalin/30% sucrose solution until sectioning. Coronal brain sections (45 μm) were cut on a cryostat and wet-mounted with 70% ethanol on glass microscope slides. Once dry, the sections were stained with 0.25% thionin to visualize neuronal cell bodies and identify cannula placements.
Data analysis
Freezing data were converted to a percentage of total observations, which is a probability estimate that is amenable to analysis with parametric statistics. These values were analyzed using analysis of variance (ANOVA), and post-hoc comparisons using Fishers LSD tests were performed after a significant overall F ratio was obtained. All data are represented as means ± SEMs.
Experiment 2: Inactivation of the BLA or CEA during US inflation does not impair US revaluation of weakly trained fear memories
Subjects and design
The subjects were 64 adult male Long–Evans rats (60–90 d old; 200–224 g; Blue Spruce) housed and handled as described in Experiment 1.
Prior to inflation, the rats were first divided into two equal groups: one group that received bilateral infusions of NBQX and a second group that received bilateral infusions of a vehicle control (VEH; 0.1M PBS; Sigma). Then, each drug group was further divided into two groups: one that received the US inflation procedure (INF) and a group that did not undergo US inflation (NoINF).
Behavioral apparatus
The behavioral apparatus was identical to that described in Experiment 1.
Surgery
One week prior to training and after having been handled for 1 wk, the rats were anesthetized and prepared for surgery as described in Experiment 1. Half of the rats that received bilateral cannulae targeting the BLA were implanted as described in Experiment 1 and the other half received bilateral cannulae implantations targeting the CEA (2.5 mm posterior to bregma, 4.3 mm lateral to the midline, and 6.9 mm ventral to the brain surface).
Conditioning, inflation, and test procedure
Fear conditioning was conducted using a limited training procedure. Rats were transported from their home cages in squads of eight and placed in the conditioning chambers (Context A). Chamber position and experimental group were counterbalanced for each squad. Rats received 10 paired presentations of a tone (10 sec, 2 kHz, 85 dB) that co-terminated with a footshock (1.0 mA, 2 sec) beginning 9 min after being placed in the chambers. There was a 60-sec ITI, and the animals remained in the boxes 60 sec after the last footshock presentation. Twenty-four hours after conditioning and prior to the inflation procedure, rats were transported to the infusion room in squads of eight from their home cages in white 5-gallon buckets. Hamilton syringes (10 μL; Harvard Apparatus) were mounted in two infusion pumps (10 syringes/pump; Harvard Apparatus) and connected to 33-gauge internal cannula (1.0 mm longer than the implanted guide cannulae) with polyethylene tubing (A-M Systems). Dummy cannulae were removed from each rat and internal cannulae were inserted into each guide cannula. Either NBQX (12 mg/mL dissolved in 0.1 M PBS, pH 7.4; Sigma) or 0.1 M PBS (same volume and rate) was infused bilaterally into the BLA or CEA (0.25 μL/side; 0.25 μL/min). One minute was allowed for diffusion of the drug into the target structure before the injectors were removed. Dummy cannulae were inserted into the guide cannulae once the injectors were removed, and 15 min after the drug infusion the rats were taken to the conditioning chambers for the inflation procedure. All rats were placed in another, novel environment (Context C) for US inflation. The inflation session consisted of exposure to five high-intensity footshocks (3.0 mA, 2 sec) beginning 6 min after being placed in the chambers. There was a 60-sec ITI, and the animals remained in the boxes 60 sec after the last footshock. Rats in the NoINF group were placed in the chamber for the same duration as the rats in the inflation groups but did not receive footshocks. Forty-eight hours after conditioning, fear to the tone was tested by placing the rats into a third novel context (Context B) and presenting 30 tone alone presentations (10 sec, 2 kHz, 85 dB, 60 sec ITI) beginning 6 min after being placed into the chambers. This experiment was designed from the use of a previous experimental protocol that did not include a context retention test; therefore, the context retention test was omitted from this experiment. Freezing behavior was measured throughout all experimental sessions.
Histology
Histology was conducted as described in Experiment 1.
Data analysis
Data analysis was performed as described in Experiment 1.
Experiment 3: Inactivation of the BLA impairs auditory fear conditioning to an intense US
Subjects and design
The subjects were 18 adult male Long–Evans rats (60–90 d old; 200–224 g; Blue Spruce) housed and handled as described in Experiment 1.
Prior to training, the rats were first divided into two equal groups: one group that received bilateral infusions of NBQX prior to training and a second group that received bilateral infusions of a vehicle control (VEH; 0.1M PBS; Sigma) prior to training.
Behavioral apparatus
The behavioral apparatus was identical to that described in Experiment 1.
Surgery
One week prior to training and after having been handled for 1 wk the rats were anesthetized and prepared for surgery as described in Experiment 1. The rats that received bilateral cannulae targeting the BLA were implanted as described in Experiment 1.
Conditioning and test procedure
Fear conditioning was conducted using a limited training procedure. Prior to conditioning, the rats were transported to the infusion room in squads of eight from their home cages in white 5-gallon buckets. Hamilton syringes (10 μL; Harvard Apparatus) were mounted in two infusion pumps (10 syringes/pump; Harvard Apparatus) and connected to 33-gauge internal cannula (1.0 mm longer than the implanted guide cannulae) with polyethylene tubing (A-M Systems). Dummy cannulae were removed from each rat and internal cannulae were inserted into each guide cannula. Either NBQX (12 mg/mL dissolved in ACSF, pH 7.4; Sigma) or ACSF (same rate and volume) was infused bilaterally into the BLA (0.5 μL/side; 0.1 μL/min). One minute was allowed for diffusion of the drug into the target structure before the injectors were removed. Dummy cannulae were inserted into the guide cannulae once the injectors were removed, and the rats were immediately taken to the conditioning chambers (Context A) for conditioning. Chamber position and experimental group were counterbalanced for each squad. Rats received five paired presentations of a tone (10 sec, 2 kHz, 85 dB) that co-terminated with a footshock (3.0 mA, 2 sec) beginning 3 min after being placed in the chambers. There was a 60-sec ITI, and the animals remained in the boxes 60 sec after the last footshock presentation. Twenty-fours hours after conditioning, all rats were placed in another novel environment (Context B) for 30 min, to reduce any generalized contextual freezing that could interfere with freezing to the tone during testing. Forty-eight hours after conditioning, fear to the tone was tested by placing the rats into Context B and presenting 30 tone-alone presentations (10 sec, 2 kHz, 85 dB, 60 sec ITI) beginning 3 min after being placed into the chambers. Seventy-two hours after conditioning, all rats were placed back into Context A for 10 min to assess contextual fear. Freezing behavior was measured throughout all experimental sessions.
Histology
Histology was conducted as described in Experiment 1.
Data analysis
Data analysis was performed as described in Experiment 1.
Acknowledgments
This research was supported by the National Institute of Mental Health Grant R01MH073655 to S.M. We thank Elizabeth Dixon, Destiny Carrillo, and Stephanie Jimenez for their assistance in behavioral testing.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1531309.
References
- Balleine BW, Killcross S. Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000a;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Price JL, Keay KA. Brain mediation of active and passive emotional coping. Prog Brain Res. 2000b;122:333–349. doi: 10.1016/s0079-6123(08)62149-4. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Holland PC, Gallagher M. Impairments in conditioned stimulus processing and conditioned responding after combined selective removal of hippocampal and neocortical cholinergic input. Behav Neurosci. 1999;113:486–495. doi: 10.1037//0735-7044.113.3.486. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Diaz-Mataix L, Debiec J, LeDoux J.E, Doyere V.Does the US reactivation trigger a reconsolidation of a CS representation in the LA?. The 38th annual meeting of the Society for neuroscience; Washington, DC. 2008. [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Nordholm L, Lodge D. The neuroprotective actions of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline (NBQX) in a rat focal ischaemia model. Brain Res. 1992;580:35–43. doi: 10.1016/0006-8993(92)90924-x. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behav Neurosci. 1992;106:518–528. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Process. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of pavlovian and instrumental outcome-specific reinforcer devaluation effects. J Neurosci. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Depaulis A, Bandler R. Different representations of inescapable noxious stimuli in the periaqueductal gray and upper cervical spinal cord of freely moving rats. Neurosci Lett. 2001;313:17–20. doi: 10.1016/s0304-3940(01)02226-1. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lees GJ. Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders. Drugs. 2000;59:33–78. doi: 10.2165/00003495-200059010-00004. [DOI] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Is there savings for Pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiol Learn Mem. 2001a;76:268–283. doi: 10.1006/nlme.2001.4042. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001b;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: Absence of a temporal gradient. Behav Neurosci. 1996a;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-d-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996b;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Maren S. Associative structure of fear memory after basolateral amygdala lesions in rats. Behav Neurosci. 2008;122:1284–1294. doi: 10.1037/a0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Ann N Y Acad Sci. 2007;1121:336–354. doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: A cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: Structure of the rat brain. Academic Press; San Diego, CA: 2004. [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:1–5. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of amygdala nuclei during acquisition of Pavlovian fear conditioning. Society for Neuroscience Abstracts. 2000;30:465–469. [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]