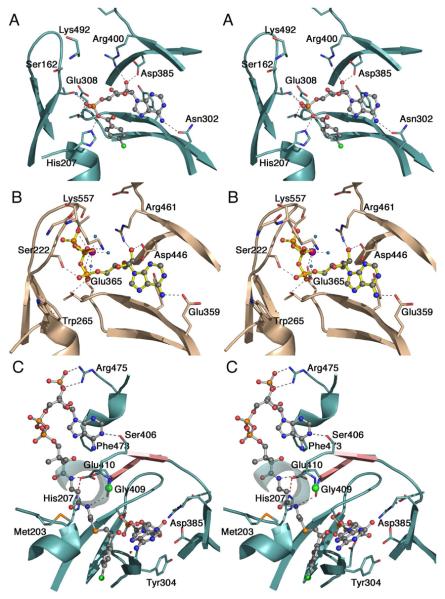
Figure 5. Active site and binding interactions of the ANL enzymes.
A. Aryl-AMP binding site of 4CBL determined in the adenylate-forming conformation (3CW8). Conserved residues that interact with the adenylate are shown. Also interacting with the α-phosphate are Thr161, from the P-loop, and Thr307. Tyr304, behind the adenine ring is unlabeled. The side chain of the A10 lysine, Lys492, was disordered beyond Cβ. B. ATP binding site of medium chain Acyl-CoA synthetase in the adenylate-forming conformation (3C5E). The interaction of the Gly- and Ser/Thr-rich P-loop with the triphosphate of ATP is clearly demonstrated. The Mg2+ ion, which bridges the b- and g-phosphates, is shown in purple. Pairs of homologous residues between mAcs and 4CBL are (mAcs listed first with 4CBL residue in parentheses): Ser222 (Thr161), Thr223 (Ser162), Trp265 (His207), Glu359 (Asn302), Tyr361 (Tyr304), Thr364 (Thr307), Glu365 (Glu308), Asp446 (Asp385), and Arg461 (Arg400). C. Binding interactions between CoA and 4CBL observed in the thioester-forming conformation (3CW9). The CoA nucleotide binds on the surface of the 4CBL enzyme, with the nucleotide ring stacking against Phe473. The pantetheine passes to the interior of the protein where the CoA thiol can attack the adenylate intermediate. Here, the thioester bond is modeled by the thioether linkage of 4-chlorophenacyl-CoA. The two strands that form the A8 loop are shown in pink and the Cα position of the universally conserved glycine at position 409 is shown with a green sphere. The β-sheet like interaction between Gly408-Gly409 and the β-alanine moiety of the pantetheine group is indicated with dashed lines. The interaction between His207 and Glu410 that stabilizes the A4 aromatic group (His207) in the new side chain position is shown with a red dashed line. The first turn of the helix at 251-265 is depicted transparently to allow the pantetheine chain to be seen.