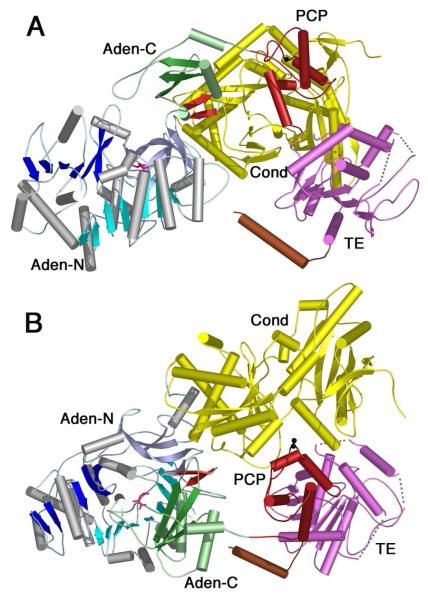
Figure 7. Crystal structure of the SrfA-C termination module from Surfactin NRPS cluster.
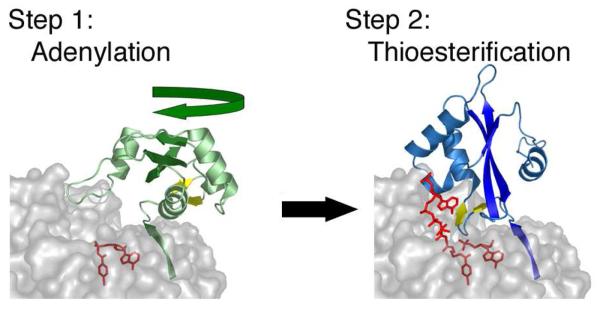
The structure contains the domains organized as Condensation-Adenylation-PCP-Thioesterase, from N- to C-terminus (2VSQ). The N-terminal domain is colored as other members of the ANL family with N-terminal domain containing β-sheets of blue and the C-terminal domain shown in green. The A8 loop is shown as the two-stranded β-sheet in red and a molecule of leucine is shown in pink in the adenylate-binding pocket. The Condensation domain (yellow), PCP domain (red) and thioesterase domain (purple) are shown. The C-terminal purification tag formed a helix that is shown in brown. The cofactor binding site, Ser1003, was mutated to an alanine and is shown in black. In panel A, the adenylation domain is oriented as for other members of the ANL family in Figures 3 and 4. The C-terminal domain most closely represents the adenylate-forming conformation, although it is opened by ~40° compared to other enzymes. In panel B, the image is rotated by ~90° around the X-axis to depict the presentation of the cofactor binding site to the condensation domain active site cleft. The pantetheine cofactor is not present in the structure yet would be unable to reach the adenylation domain active site without a large conformational change.