Abstract
There are declines in the protein expression of the NR2B (mouse ε2) and NR1 (mouse ζ1) subunits of the N-methyl-D-aspartate (NMDA) receptor in the cerebral cortex and hippocampus during aging in C57BL/6 mice. This study was designed to determine if there is a greater effect of aging on subunit expression and a stronger relationship between long-term spatial memory and subunit expression within the synaptic membrane than in the cell as a whole. Male, C57BL/6JNIA mice (4, 11 & 26 months old) were tested for long-term spatial memory in the Morris water maze. Frontal cortex, including prefrontal regions, and hippocampus were homogenized and fractionated into light and synaptosomal membrane fractions. Western blots were used to analyze protein expression of NR2B and NR1 subunits of the NMDA receptor. Old mice performed significantly worse than other ages in the spatial task. In the frontal cortex, the protein levels of the NR2B subunit showed a greater decline with aging in the synaptic membrane fraction than in the whole homogenate, while in the hippocampus a similar age-related decline was observed in both fractions. There were no significant effects of aging on the expression of the NR1 subunit. Within the middle-aged mouse group, higher expression of both NR2B and NR1 subunits in the synaptic membrane was associated with better memory. In the aged mice, however, higher expression of both subunits was associated with poorer memory. These results indicate that aging could be altering the localization of the NR2B subunit to the synaptic membrane within the frontal cortex. The correlational results suggest that NMDA receptor functions, receptor subunit composition, and/or the environment in which the receptor interacted in the hippocampus were not the same in the old animals as in younger mice and this may have contributed to memory declines during aging.
Keywords: Prefrontal cortex, Hippocampus, NR2B (ε2), NR1 (ζ1), spatial, learning
Memory is one of the earliest of the cognitive functions to show declines during aging (Albert and Funkenstein, 1992). Memory deficits associated with aging are seen in humans and non-human primates (see reviews (Gallagher and Nicolle, 1993, Gallagher and Rapp, 1997)), dogs (Head et al., 1995) and rodents (Gage et al., 1984, Rapp et al., 1987, Barnes, 1988, Zyzak et al., 1995). One type of memory that is important for how individuals cope with their environment is spatial memory. Humans show 30% to 80% drops in performance in spatial memory tasks as they age (Evans et al., 1984, Moore et al., 1984, Sharps and Gollin, 1987, Kirasic and Bernicki, 1990, Cherry and Park, 1993, Moffat and Resnick, 2002). Mice and rats also exhibit deficits in spatial memory during aging, in both short-term (working) and long-term (reference) memory (Barnes, 1979, Gage et al., 1984, Pelleymounter et al., 1987, Rapp et al., 1987, Barnes, 1988, Magnusson, 1998a, 2001, Magnusson et al., 2003). A long-term spatial memory task was used in the present study to assess age-related memory declines (Magnusson et al., 2007).
The N-methyl-D-aspartate (NMDA) receptor, a subtype of glutamate receptors, is particularly important in neuronal plasticity (Morris and Davis, 1994). NMDA antagonists impair formation of long-term memory (Morris et al., 1986, Alessandri et al., 1989, Mondadori et al., 1989, Heale and Harley, 1990) and block the initiation of long-term potentiation (LTP) in the hippocampus (Harris et al., 1984, Morris et al., 1986, Bashir et al., 1991) and neocortex (Artola and Singer, 1994). These studies suggest that detrimental changes to the NMDA receptor during the aging process should have a negative impact on memory.
Aging animals do exhibit declines in NMDA receptor binding densities and functions, including memory. NMDA-stimulated release of transmitters is decreased with increasing age (Gonzales et al., 1991, Pittaluga et al., 1993). LTP is also altered in aged rodents (Barnes and McNaughton, 1985, Deupree et al., 1993). Age-related declines in binding of glutamate and [(±)-2-carboxypiperazin-4-yl] propyl-1-phosphonic acid (CPP) to NMDA binding sites have been reported in mice, rats, dogs, and monkeys (Kito et al., 1990, Pelleymounter et al., 1990, Tamaru et al., 1991, Wenk et al., 1991, Magnusson, 2000, Magnusson et al., 2000). Humans also exhibit declines with increased age in binding of [3H]MK801 to the NMDA receptor complex in the frontal cortex (Piggott et al., 1992). Declines during aging in NMDA binding site densities within both frontal cortical regions (Magnusson, 1998a) and in the hippocampus (Pelleymounter et al., 1990, Magnusson, 1998a) have been correlated with poor performance in long-term memory tasks, such as those utilizing the Morris water maze. The different binding sites on the NMDA receptor complex do not all show the same declines in binding density within the brains of C57BL/6 mice during aging (Magnusson, 1995, 1998b). This suggests that different portions, or possibly subunits, of the receptor are differentially affected by aging.
Functional subunits of the NMDA receptor complex have been cloned for rats (Moriyoshi et al., 1991, Monyer et al., 1992, Ishii et al., 1993) and mice (Ikeda et al., 1992, Kutsuwada et al., 1992, Meguro et al., 1992, Yamazaki et al., 1992). Although, the original NMDA receptor subunit families that were identified in mice were named zeta and epsilon (Ikeda et al., 1992, Kutsuwada et al., 1992, Meguro et al., 1992, Yamazaki et al., 1992), NR1 and NR2 are the more commonly used names among other species (Moriyoshi et al., 1991, Monyer et al., 1992, Ishii et al., 1993) and will be used in this paper. The NR1 (mouse ζ1) subunit has the same distribution as NMDA-displaceable [3H]glutamate binding (Moriyoshi et al., 1991, Meguro et al., 1992, Nakanishi, 1992). The four members of the NR2 family of subunits, NR2A-D (mouse ε1-4) (Ikeda et al., 1992, Kutsuwada et al., 1992, Meguro et al., 1992) each provide different agonist/antagonist affinities to NR1/NR2 heteromeric receptors (Kutsuwada et al., 1992, Yamazaki et al., 1992). These NR2 subunits also produce different gating behaviors, responses to Mg++, and I/V curves (Monyer et al., 1992, Ishii et al., 1993).
There are effects of aging on some of the subunits of the NMDA receptor. The density of mRNA expression for the NR2B subunit declines with increasing age in the cerebral cortex and dentate gyrus of male C57BL/6 mice (Magnusson, 2000). These changes in NR2B mRNA expression correlate significantly with age-related changes in binding of agonist to NMDA sites across both cortical and hippocampal regions (Magnusson, 2000) and within frontal cortical regions (Magnusson, 2001). Some of the age-related decline in the NR2B subunit mRNA and protein expression in the adult brain appears to be due to a continuation of the decline that begins during development (Ontl et al., 2004). This pattern is also seen functionally in electrophysiological responses of NMDA receptors across development and aging in rats (Yang et al., 2008). There is also an overall decrease during aging in mRNA expression of the NR1 subunit across cortical and hippocampal regions, but to a lesser degree than seen with the NR2B subunit (Magnusson, 2000).
C57BL/6 mice show significant declines during aging in the protein expression of NR2B and NR1 subunits in homogenates from the whole cerebral cortex (Magnusson et al., 2002) and in crude synaptosomes from frontal cortex (Magnusson et al., 2007). Significant declines during aging in protein expression in hippocampal homogenates are also seen in the NR1 and NR2B subunits in both mice (Magnusson et al., 2002) and Fisher 344 rats (Eckles-Smith et al., 2000, Clayton and Browning, 2001). Fisher344xBrown Norway F1 (F344BN) rats show significant declines in protein expression of the NR2B and NR2A subunits, but not the NR1 subunit in the hippocampus (Sonntag et al., 2000). Homogenates of only frontal cortex from mice show a significant age-related decrease in the protein expression of the NR2B subunit alone (Ontl et al., 2004). In a separate study using crude synaptosomes from mouse frontal cortex (Magnusson et al., 2007), the declines in the NR2B subunit protein expression were greater than seen in the homogenates (Ontl et al., 2004). One aim of the current study was to determine whether there are greater changes during aging in the expression of NMDA receptor subunits in the synaptic membrane than in the whole tissue. We subfractionated the tissue into synaptosomal membrane and light membrane (containing Golgi apparatus and endoplasmic reticulum) fractions and compared these to the total homogenate (Dunah and Standaert, 2001). We also addressed the hypothesis that the relationships between the NR2B and NR1 subunits of the NMDA receptor and memory ability would be strongest with the protein expressed in the synaptic membrane, since those receptors should be directly involved in neurotransmission.
EXPERIMENTAL PROCEDURES
Animals
Thirty-four, male C57BL/6JNIAHSD mice (National Institute on Aging, Bethesda, MD, USA) from 3 different age groups (4, 11, and 26 months of age at time of behavioral testing and euthanasia) were used. Mice were ad libitum-fed and housed under 12/12 hour light/dark conditions for 1.5 months prior to and during behavioral testing at University of Idaho. All animal procedures were approved by the Institutional Animal Care and Use Committee at University of Idaho and conformed to NIH guidelines.
Behavioral testing
Apparatus
A 1.2 meter diameter metal tank was filled with water (~18–20°C), made opaque with non-toxic paint, to 1 cm above the level of the platform. The spatial cues consisted of posters and geometric figures located high on the walls of the room and tank. There were platform positions in the center of each quadrant and 3 positions that each differed from all other positions in their distance from the wall. One of three entry points, within the non-platform quadrants, were randomly assigned for each trial. Trials were videotaped with a Panasonic color CCD camera and VCR and path tracings were captured and analyzed with the use of a SMART Video Tracking System (San Diego Instruments, San Diego, CA, USA) and a Dell computer. Pretraining occurred during the 2 days prior to place training and consisted of an animal swimming for 60 seconds in the tank without the platform and then being trained to remain on a platform located in the center of the tank for 30 seconds each day. This platform location was not the one used for the long-term memory testing. Mice then underwent place training and probe testing to assess long-term memory, followed by control cued trials.
Place training & probe trials
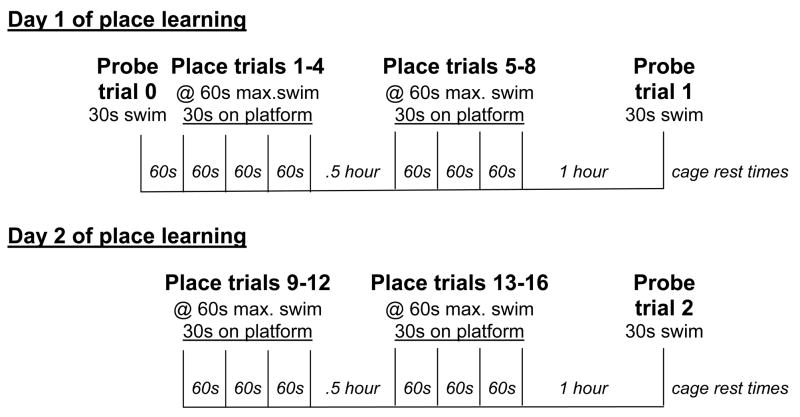
A two-day task for long-term spatial memory was adapted for mice (Magnusson et al., 2007) (Fig. 1) from a one-day task developed by Gallagher and coworkers (Berry et al., 1997). On day 1, mice underwent one naïve probe trial, one block of 4 place training trials with a 60 second inter-trial interval in a cage between each trial, 30 minutes of cage rest, another block of 4 place training trials with 60 second inter-trial intervals, 1 hour of cage rest, and a final probe trial (Fig. 1). Day 2 was the same except that there was no probe trial at the beginning of training (Fig. 1). The platform remained in the same quadrant (NE) for all the place training trials. During each place training trial, a mouse was placed in the tank facing the wall, and was allowed to search for the platform for 60 seconds. If the animal did not find the platform by the end of that time, it was lead to the platform. The mouse remained on the platform for 30 seconds before being removed to a cage to rest. The probe trials were designed to assess the animal’s memory or spatial bias for the platform location (Gallagher et al., 1993). The naïve probe trial was used to determine if there were any pre-existing biases and to be able to show improvement in subsequent probe trial performances. The platform was not present during the probe trials and the animal was allowed to search for 30 seconds and then was removed to a drying cage under heat lamps.
Fig. 1.
Diagram of the protocol for long-term spatial memory testing over a two-day period, including both place training and probe trials. s, seconds; max., maximum.
Cued trials
On the day following long-term memory testing, the cued trials were run. The platform was submerged, but was marked by a 20.3 cm tall support with a flag. There were six different platform positions used for the six cued trials. The platform locations for the cued trials were as follows: 1 – South (close to the wall), 2 – Center of tank, 3 – NE, 4 – North (half of the distance between a quadrant position and the wall), 5 - SE and 6 - NW. For each trial, the animal was placed into the tank, facing the wall at one of the entry points, and was allowed to search for the platform for 60 seconds. If the animal did not find the platform by the end of that time, it was lead to the platform. Each animal was tested at one platform position and then the platform was moved. This continued until all 6 positions were used. Cued trials were designed to test visual acuity, physical ability, and motivation for the task.
Analysis
Average distance was calculated by the SMART video tracking system according to the method of Gallagher et al. (Gallagher et al., 1993). Briefly, the animal’s distance from the platform, or proximity, was measured by the computer every frame (0.2 sec) for the duration of the animal’s swim. The SMART system added the distance measures together and divided by the number of samples to obtain an average distance for the trial. Cumulative proximity and correction for start position were calculated and applied with the use of Excel software (Microsoft Corp., Seattle, WA, USA). The average distance was multiplied by the number of samples in the trial in order to obtain a cumulative proximity for each animal’s place and cued trials. With the use of an Excel macro, the average speed, starting point, platform center point and Pythagorean theorem (a2 + b2 = c2) were used to calculate the cumulative proximity for an ideal path. This ideal cumulative proximity was subtracted from the cumulative proximity for the same trial to obtain a corrected cumulative proximity. For the probe trials, the corrected cumulative proximity for the trial was divided by the corrected sample number (obtained by subtraction of the sample number from the ideal path from the trial sample number) in order to obtain a corrected average proximity. Learning index scores were calculated from the probe trial data as previously described (Gallagher et al., 1993). The mean corrected average proximity for the young mice in the naïve probe trial (probe trial 0) was divided by the mean average proximity for the young mice in each separate probe trial in order to obtain a multiplier for each probe trial. The multipliers obtained were as follows: 1.00, 1.189, and 1.329 for probe trials 0–2, respectively. For each mouse, the average proximity for each trial was multiplied by the respective multipliers for each trial and the products were summed to obtain a learning index score for that mouse.
For both cumulative and average proximity and learning index scores, higher values represented poorer search accuracy and lower values indicated better search accuracy. Corrected proximity measures were used to assess memory in these studies because they are less influenced by swim speed differences than latency to reach the platform (Gallagher et al., 1993, Magnusson, 1998a, Magnusson et al., 2007), more sensitive to alternative, non-spatial strategies (Gallagher et al., 1993), and more sensitive to group differences than other measures, such as quadrant or zone dwell time and platform crossings (Maei et al., 2009). The learning index score provides similar information to traditional measurements of time spent in the correct quadrant, but has the added advantage of providing a single value that can represent the spatial bias in multiple probe trials and also reflect the learning curve by being weighted to reward those animals who acquire the task faster (Gallagher et al., 1993).
Tissue preparation
Within 2 hours of the end of cued testing, the mice were euthanized by exposure to CO2, followed by decapitation. The brains were removed, frozen on dry ice, and stored in a −80°C freezer until dissection. The brain was warmed to −20°C, placed in a plastic brain mold (Braintree Scientific, Braintree, MA, USA) on ice, and cut in the coronal plane. The rostral 3 mm of both hemispheres of the cortex were dissected and used for semi-quantitation of the protein expression of the NR1 and NR2B subunits of the NMDA receptor and glyceraldehyde 3-phosphate dehydrogenase (GADPH; loading control) by Western blotting. These frontal cortex dissections included orbital, limbic, insular, cingulate, primary and association motor and sensory cortices (Franklin and Paxinos, 1997). Olfactory bulb, caudate nuclei and brainstem were dissected away from the cortex and discarded. The hippocampus was isolated from the cerebral cortex, removed, and used to analyze the same proteins described above. The remaining caudal cortices (including parietal, occipital and temporal cortices) from the 4-month-old mice were used to produce standard curves for protein analysis. Brains were randomly assigned to an assay group, which consisted of multiple representatives of each age. The brains within an assay group were processed and assayed at the same time.
Preparation of homogenate and light and synaptosomal membrane fractions
The frontal cortex and hippocampus were homogenized separately. A portion of this homogenate was subfractionated into synaptosomal membrane and light membrane fractions as previously described (Dunah and Standaert, 2001) with some modifications. The caudal cortices from the 4-month-old mice were processed along with the samples of frontal cortex and hippocampus and then combined prior to the protein assay for use as cortical standards. Dissected brain regions were placed in Dounce homogenizers containing ice-cold TE buffer (10mM Tris HCl, 1mM EDTA, 1 mM EGTA, pH 7.4) plus a protease inhibitor cocktail (P.I., 2 μl/ml buffer; Sigma, St. Louis, MO, USA) and 320 mM sucrose and homogenized by hand. An aliquot of this homogenate was saved and centrifuged at 13,000 X g for 42 minutes in a Savant μSpeedfuge SFR13K refrigerated centrifuge with RSR-20 rotor (Thermo Fisher Scientific, Waltham, MA, USA). The pellet was resuspended in TE buffer plus P.I. and was used to represent the homogenate. The remaining original homogenates were centrifuged at 1000 X g for 3 minutes in the Savant centrifuge. The pellet (P1) was discarded. The supernatant (S1) was centrifuged at 9000 X g for 11 minutes in the Savant centrifuge to obtain supernatant (S2) and pellet (P2). The S2 supernatant was centrifuged at 164,170 X g in a Beckman Optima TL tabletop ultracentrifuge with a TLA 120.1 rotor for 24 minutes to obtain the light membrane fraction (P3). The P3 fraction was resuspended in TE buffer plus P.I. The P2 pellet (crude synaptosomal fraction) was reconstituted in TE buffer plus P.I., as described above, and 35.6 mM sucrose and incubated on ice for 30 minutes to induce hypo-osmotic lysis. The lysed P2 pellet was centrifuged at 13,000 X g for 27 minutes in the Savant centrifuge and the pellet (LP1) was resuspended in TE buffer plus P.I. to obtain the synaptosomal membrane fraction. Each resuspended fraction received an equal volume of 2X lysis buffer (10mM Tris HCl, pH 6.8; 20% glycerol, 2 % sodium dodecyl sulfate (SDS)) and was tested for protein concentration using the DC Protein Assay kit (Bio-Rad Laboratories; Hercules, CA, USA). Each fraction was diluted in 1X lysis buffer to 2 to 5 mg/ml protein, aliquoted and stored at −80°C.
Western blots
Western blotting was performed as previously described (Dunah and Standaert, 2001) with some modifications. The homogenate and different fractions were each assayed separately. The caudal cortex used for standards was processed the same as the samples being assayed (e.g., homogenate of caudal cortex used as standards for homogenate samples). Aliquots of standards and samples were thawed, diluted in 1X lysis buffer, 2 mM dithiothreitol, 2 mg/ml bromophenol blue and boiled for 5 minutes. Four different amounts of protein (1.5 – 12 μg) from the caudal cortex were loaded on each gel as standards along with 6–10 μg of protein from the frontal cortex or hippocampus from each animal in one assay group. SDS-polyacrylamide gels (7.5%) were run in triplicate and transferred to Sequi-Blot Polyvinylidene Fluoride (PVDF) membrane (Towbin et al., 1979). The positions of representatives for each age group were alternated across the gel. Strips of each gel containing the appropriate molecular weight range for each protein of interest were cut and blotted separately. The membranes were blocked in a blocking buffer (Odyssey Blocking Buffer (Li-Cor Biotechnology, Lincoln, NE, USA) diluted 1:1 with Tris-buffered saline (TBS; 20 mM Tris-HCl, 140 mM NaCl, pH 7.2)) for 1 hour at room temperature and were incubated overnight at 4°C in blocking buffer containing primary antibodies. The antibodies to identify the NR1 and NR2B subunits of the NMDA receptor were purchased from Zymed (So. San Francisco, CA, USA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), respectively. The GAPDH antibody was obtained from Calbiochem (EMD Chemicals, Inc., Gibbstown, NJ, USA). The membranes were rinsed 5 times for a total of 35 minutes in TBS with 0.05% Tween-20 (TBS-T), incubated with infrared dye-conjugated secondary antibody (IR800dye, Rockland, Gilbertsville, PA, USA; Alexa Flour 680, Molecular probes (Invitrogen, Carlsbad, CA, USA)) diluted in blocking buffer plus 20% SDS for 1 hour at room temperature, rinsed 5 times for 35 minutes total in TBS-T and rinsed 1 time for 15 minutes in TBS. Membranes were scanned by an Odyssey scanner (Li-Cor Biotechnology, Lincoln, NE, USA) and integrated intensities of bands were obtained with Odyssey image analysis software (Li-Cor Biotechnology, Lincoln, NE, USA). Standard curves were obtained with the use of Prism software (GraphPad Software, San Diego, CA, USA) using a linear regression fit. Sample bands were analyzed, interpolated from the standard curve and expressed as μg cortical protein equivalents. Sample bands that had densities within the saturated portion of the standard curve were not used. The μg cortical protein equivalents for NR1 and NR2B subunits were divided by the μg cortical protein equivalents for GAPDH from the same gel lane to correct for differences in gel loading. Each NR2B and NR1 subunit/GAPDH value within the homogenate or fraction was then expressed as a percentage of the average 4 month old expression for the respective homogenate or fraction in order to allow better comparisons between whole tissue and fractions.
Data analysis
For place training trials, the data from each consecutive pair of trials were averaged to obtain a single value for blocks of two trials before performing statistical analysis. Statistical analysis for behavior was performed by repeated measures analysis of variance (ANOVA; age X trial), followed by Fisher’s protected least significant difference (LSD) post-hoc test where applicable. Age-related differences in protein expression for homogenate or fractions and protein were analyzed by one-way ANOVA (age), followed by Fisher’s protected LSD where indicated. ANOVA and post-hoc tests were performed with the use of Statview software (SAS Institute, Inc., Cary, N.C., USA).
Correlations between behavioral measurements and protein expression were examined using Pearson correlation coefficients. The behavioral measurement used for the place training trials was the mean cumulative proximity for all blocks of place trials. The learning index score was used for the probe trials. Correlations were performed with individuals within an age group. To correct for the number of comparisons, a recently developed method, p_ACT version 1.0 (Conneely and Boehnke, 2007) was run using R statistics software version 2.6.1 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria) with the package mvtnorm version 0.8–1 (Genz et al., 2007). This method of adjustment adjusts for p-values of different correlation tests sequentially and is based on a procedure described previously (Holm, 1979). The correction was applied by setting the value of alpha to .05 to comparisons within a hypothesis and/or comparisons potentially related to each other.
RESULTS
Behavioral testing results
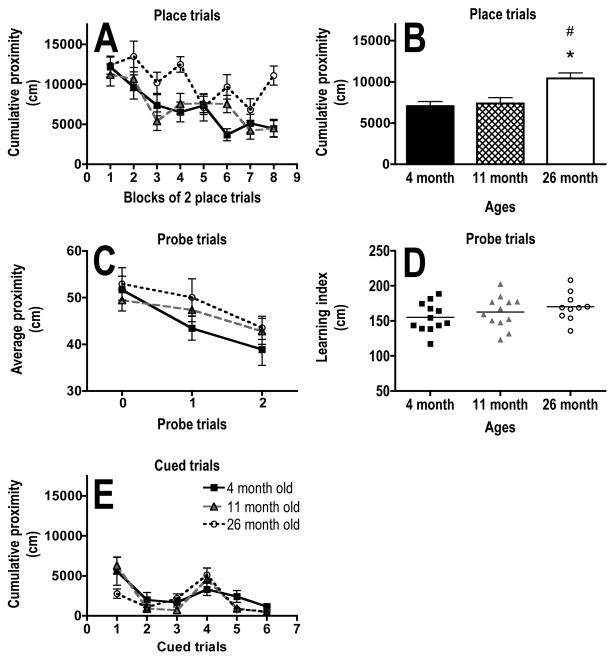
There was a significant main effect of age (F (2,31) = 7.5, P = .002) and trial block (F (7,217) = 11.6; P <.0001) on corrected cumulative proximity in the place training trials (Fig. 2A, B). The 26-month-old mice had significantly higher corrected cumulative proximity than both the 4- (P = .002) and 11- (P = .017) month-old mice when data was averaged across all blocks of place trials (Fig. 2B). The 4- (P = .0003) and 11- (P = .0002) month-old mice had significantly lower corrected cumulative proximities on the last place trial block as compared to the first (Fig. 2A). The 26 month olds did not show any significant difference in corrected cumulative proximities between the first and last trial blocks (P = .41; Fig. 2A).
Fig. 2.
Effects of age on long-term memory and cued tasks in the Morris water maze. A, B) Graphs showing the performance, measured as cumulative proximity in cm, of 4-, 11- and 26-month-old mice within blocks of 2 place training trials (A) and averaged across all place training trials (B). C) Graph showing the performance, measured as average proximity in cm, of 4-, 11-and 26-month-old mice within individual probe trials. D) Individual learning index scores with the horizontal bar indicating the mean for each age group. E) Graph showing the performance, measured as cumulative proximity in cm, of 4-, 11- and 26-month old mice within cued trials. Proximity measures were corrected for start position. * indicates P < .05 for difference from 4 month olds and # indicates P < .05 for difference from 11 month olds (ANOVA and Fisher’s protected least significant difference). N = 12 for 4 and 11 months olds. N = 10 for 26 month olds. Error bars represent SEM.
There was no significant main effect of age (F(2,31) = 1.3, P = .29), but a significant main effect of trial (F(2,62) = 8.5, P = .0006) on corrected average proximity in the probe trials (Fig. 2C). The 4-month-old mice had a significantly lower corrected average proximity in the last probe trial (P2) as compared to the naïve trial (P0; P = .016; Fig. 2C). The 11- and 26-month-old mice did not show a significant difference in corrected average proximities between the first and last probe trials (P = .07; Fig. 2C). There was no significant effect of age on the learning index score (P = .26; Fig. 2D). There was no significant main effect of age (F(2,31) = .8, P = .46) on cumulative proximity in the cued control trials (Fig. 2E).
Protein expression
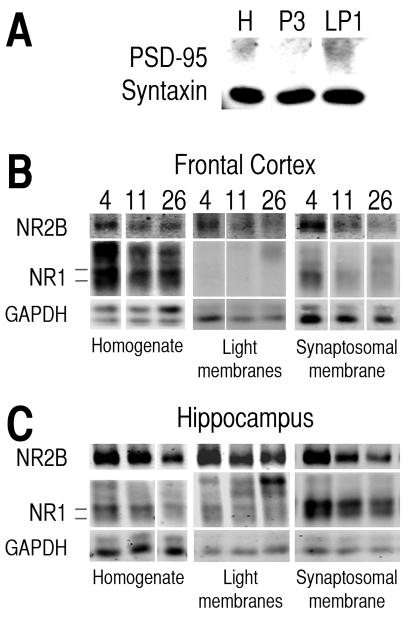
The synaptosomal membrane fraction (LP1) showed more expression of post-synaptic density protein-95 (PSD-95) than the homogenate (H) and light membrane (P3) fractions (Fig. 3A). Syntaxin was found in all three fractions (Fig. 3A). In some homogenates and fractions, there were 4 bands for the NR1 subunit between 110 and 130 kDa (Fig. 3B, C). The two bands near 110 kDa were analyzed for this study. The upper bands were not consistently present across animals and did not appear to be associated with specific ages.
Fig. 3.
Representative bands of protein from different cellular fractions of frontal cortex and hippocampus. A) Representative bands of post-synaptic density-95 (PSD-95) and syntaxin proteins in homogenate (H), light membrane (P3), and synaptosomal membrane (LP1) fractions from caudal cortex. B, C) Representative bands of NR2B and NR1 subunit proteins of the NMDA receptor and GAPDH protein from homogenate, light membrane fraction and synaptosomal membrane fraction for frontal cortex (B) and hippocampus (C) from 4-, 11- and 26-month-old mice. Note: The scanned bands were in color when analyzed. The images were converted to grayscale for publication purposes. For each protein and fraction shown, the images of bands representing the three different age groups were all obtained from the same gel and were adjusted for brightness and contrast as a group.
NR2B subunit
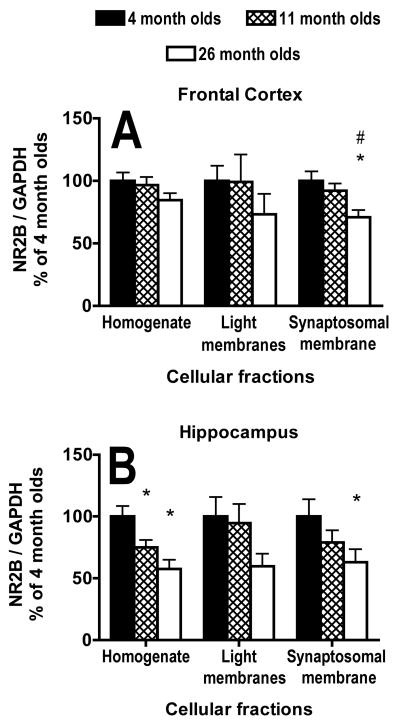
There was no significant main effect of age on NR2B subunit/GAPDH expression in either the homogenate (F(2,31) = 1.5, P = .23) or light membrane fraction (F(2,31) = .7; P = .50) in the frontal cortex (Fig. 3B,4A). There was a significant main effect of age on the expression of the NR2B subunit/GAPDH in the synaptosomal membrane fraction from the frontal cortex (F(2,31) = 5.0; P = .01; Fig. 3B,4A). Both the 4- (P = .004) and 11- (P = .03) month-old mice had significantly higher subunit expression than the 26 month olds (Fig. 3B,4A).
Fig. 4.
Effects of age on protein expression of the NR2B subunit of the NMDA receptor in different cellular fractions. A, B) Graphs of protein expression of the NR2B subunit of the NMDA receptor from homogenate and light and synaptosomal membrane fractions; corrected for loading by GAPDH protein expression within the same lane and expressed as a percentage of the average for 4-month-olds, in the frontal cortex (A) and hippocampus (B) from 4-, 11- and 26-month-old mice. * indicates P < .05 for difference from 4 month olds and # indicates P< .05 for difference from 11 month olds (ANOVA and Fisher’s protected least significant difference). N = 12 for 4 and 11 months olds. N = 10 for 26 month olds. Error bars represent SEM.
There was a significant main effect of age on NR2B subunit/GAPDH expression in the homogenate (F(2,31) = 8.1, P = .0015) from the hippocampus (Fig. 3C,4B). The 4-month-old mice had significantly higher NR2B subunit/GAPDH expression than both the 11- (P = .02) and 26- (P = .0004) month-old mice in the hippocampal homogenate (Fig. 3C,4B). There were no significant main effects of age on the light membrane (F(2,31) = 2.2, P = .13) or synaptosomal membrane (F(2.31) = 2.4, P = .10) fractions from the hippocampus (Fig. 3C,4B). The 4-month-old mice had significantly greater NR2B subunit/GAPDH expression in the synaptosomal membrane fraction from the hippocampus than the 26 month olds (P = .036; Fig. 3C,4B).
NR1 subunit
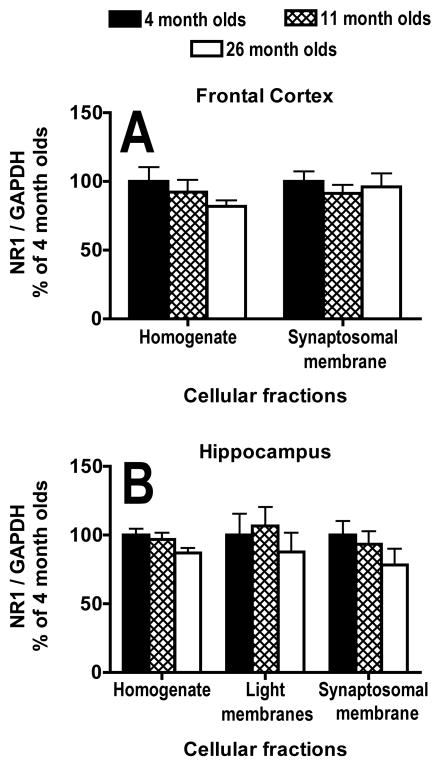
There was no significant main effect of age on NR1 subunit/GAPDH expression in either the homogenate (F(2,31) = 1.1, P = .35) or synaptosomal membrane fraction (F(2,31) = .3, P = .72) of the frontal cortex (Fig. 3B,5A). The bands present at 110 kDa on blots from the light membrane fraction of the frontal cortex were too light to analyze (Fig. 3B). There was no significant main effect of age on NR1 subunit/GAPDH expression in either the homogenate (F(2,31) = 2.1, P = .14), light membrane fraction (F(2,31) = .4, P = .67) or synaptosomal membrane fraction (F(2,31) = 1.0, P = .37) from the hippocampus (Fig. 3C,5B).
Fig. 5.
Effects of age on protein expression of the NR1 subunit of the NMDA receptor in different cellular fractions. A, B) Graphs of protein expression of the NR1 subunit of the NMDA receptor from homogenate, light membrane (hippocampus only) and synaptosomal membrane fractions; corrected for loading by GAPDH protein expression within the same lane and expressed as a percentage of the average for 4-month-olds, in the frontal cortex (A) and hippocampus (B) from 4-, 11- and 26-month-old mice. N = 12 for 4 and 11 months olds. N = 10 for 26 month olds. Error bars represent SEM.
Correlations between behavior and subunit expression in old mice
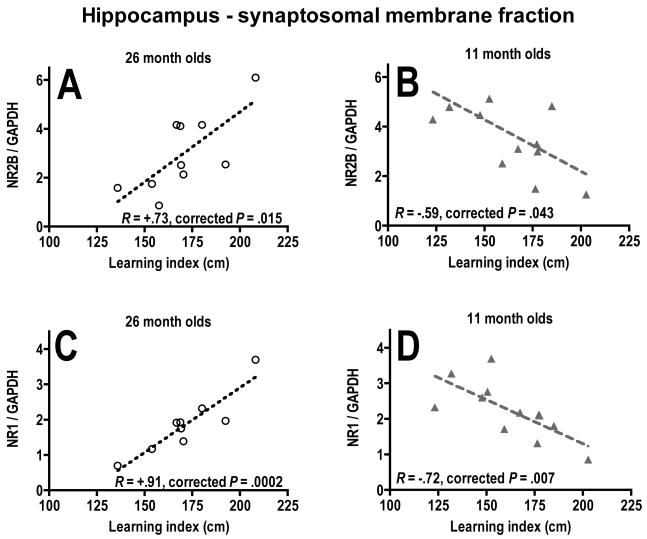
There was no significant correlation (P = .44) between the mean corrected cumulative proximities for all place training trial blocks and the learning index scores obtained from all probe trials for the old mice, so each behavioral measure was analyzed separately. There were no significant correlations found in the frontal cortex (Table 1). There were significant positive correlations between both NR2B and NR1 subunits/GAPDH in the synaptosomal membrane fraction from the hippocampus of 26-month-old mice and learning index scores (Fig. 6A, C; Table 1). There was also a positive correlation between NR1 subunit/GAPDH in the homogenate fraction from the hippocampus of 26-month-old mice and mean cumulative proximity in the place training trials (Table 1). Because higher proximities and learning index scores indicated more time spent away from the platform location, a positive correlation with these measurements reflected a negative correlation between subunit expression and memory in the 26-month olds. The 4- and 11-month-old mice were then examined for the same comparisons. The only significant correlations were negative correlations in the 11-month-old mice between the expression of both NR2B subunit and NR1 subunit/GAPDH in the synaptosomal membrane fraction and learning index scores (Fig. 6B, D).
Table 1.
Pearson correlation coefficients (R) for protein expression of NMDA receptor subunits in homogenate or synaptosomal membrane and measurements of long-term spatial memory in 26-month-old mice.
| Place trials (cum. prox.) |
Probe trials (learning index) |
|||
|---|---|---|---|---|
| Homogenate |
Synaptosomal membrane |
Homogenate |
Synaptosomal membrane |
|
| Frontal Cortex | ||||
| NR2B/GAPDH | +.05 | +.05 | +.51 | +.43 |
| NR1/GAPDH | +.05 | +.70 | −.20 | −.12 |
| Hippocampus | ||||
| NR2B/GAPDH | +.52 | +.05 | −.43 | +.73* |
| NR1/GAPDH | +.76* | −.18 | −.33 | +.91** |
corrected P< .05;
corrected P< .01 (p_ACT correction for multiple comparisons); Correlations include individual mice from the 26 month old group. N = 9–10. Place trials (cum. prox.); corrected cumulative proximities averaged across all place trials. Probe trials (learning index), learning index calculated from all probe trials.
Fig. 6.
Relationships between long-term memory and protein expression of NMDA receptor subunits. Correlation graphs of NR2B subunit/GAPDH (A, B) and NR1 subunit/GAPDH (C, D) protein expression in the synaptosomal membrane fraction of the hippocampus versus learning index scores for probe trials for 26-month-old mice (A, C) and 11-month-old mice (B, D). corrected P, P values corrected by p_ACT statistical method.
DISCUSSION
There were 4 major findings in this study. 1) The results of this study support the hypothesis that there was a greater decline during aging in the protein expression of the NR2B subunit within the synaptic membrane of the frontal cortex than in the tissue as a whole. 2) In the hippocampus, the expression of the NR2B subunit showed similar declines between whole homogenate and synaptic membranes. 3) Both the NR2B and NR1 subunits of the NMDA receptor within the synaptic membranes of the hippocampus in the old mice showed a negative relationship to performance in the long-term memory task; i.e., high expression levels of the two subunits were found in the worst performers. 4) In the middle-aged mice, this relationship was reversed, with high expression of both subunits associated with better performance.
The significant decline in place learning performance between the 4- and 26-month-old mice was similar to our previous results with 3 and 26 month old C57BL/6 mice using the same 2 day task (Magnusson et al., 2007). These results were also similar to performance differences seen in a 12-day reference memory task using this strain of mice (Magnusson, 1998a, 2001, Magnusson et al., 2003). All ages of mice in this study performed similarly in the cued control task, so the age-related deficits seen in place training were most likely to reflect problems with long-term spatial memory.
Probe trials can indicate the spatial bias for the former platform location developed by the animal (Gallagher et al., 1993). There were no significant differences in overall performance between the different age groups in the probe trials in the current study. This is in contrast to our previous results in which 26 month old C57BL/6 mice performed significantly worse than 3 month olds in a similar task (Magnusson et al., 2007). The 4-month-old mice in this study did not show as much of an improvement between the naive and last probe trial (25% improvement) as the 3 month old mice did (42% improvement) in the previous study (Magnusson et al., 2007). This suggests that the young mice may not have developed a strong enough spatial bias in the present study. Old mice showed similar improvements in both studies (18% versus 13.4% (Magnusson et al., 2007)). The young mice in the current study were 1.5 months older than in the previous study (Magnusson et al., 2007) and behavioral testing was performed at different institutions with slightly different room organizations. However, the similar results for the place training trials between the two studies suggest that these factors did not affect all aspects of performance. The young mice in the current study did show a significant improvement between the first and last probe trials that wasn’t seen in the middle-aged and old mice, so there was some indication of an age difference in spatial memory reflected in the probe trials.
Many other studies showing memory declines during aging in mice report the use of between 1 and 12 days to assess long-term memory (Forster et al., 1996, Haroutunian et al., 1996, Frick et al., 2000, Buhot et al., 2003, Frick and Fernandez, 2003, Frick et al., 2003, Trinchese et al., 2004, van Praag et al., 2005). It should be noted, however, that Calhoun and co-workers found no effect of aging on male C57BL/6 mice performing a long-term memory task in the water maze for 5 days (Calhoun et al., 1998). Thus, the length of acquisition doesn’t seem to adequately account for differences in sensitivity in different studies. In addition, although three out of four experiments in our laboratory using a 12-day training period showed that old C57BL/6 mice were impaired in both place training and probe trials. (Magnusson, 1998a, 2001, Das and Magnusson, 2008), one study showed deficits in only the place training trials (Magnusson et al., 2003). This suggests that the place training trials may be more sensitive than probe trials for detecting age-related differences in long-term memory.
The synaptosomal membrane fraction (LP1) showed a higher expression of the post-synaptic density protein (PSD-95) than the whole homogenate or light membrane fraction (P3). This was similar to the results reported within the rat cortex with the use of the same subfractionation protocol (Dunah and Standaert, 2001). The presence of PSD-95 indicated that the synaptosomal membrane fraction was enriched in a protein associated with the synaptic membrane (Sierralta and Mendoza, 2004), which contains the proteins involved in receptor anchoring, signaling and modulation (Kennedy, 1993, Ziff, 1997). Syntaxin, a protein that is involved in the docking of synaptic vesicles (Bennett et al., 1992, Barinaga, 1993), was found in all three fractions in both the frontal cortex and hippocampus (not shown) in this study, similar to that seen in the striatum of rats (Dunah and Standaert, 2001). The extremely light signal for the NR1 subunit in the light membrane fraction in the frontal cortex was also similar to that reported in the rat cerebral cortex (Dunah and Standaert, 2001). These results indicate that the subfractionation technique produced the light membrane and synaptosomal membrane fractions as expected. The PSD-95 and syntaxin results suggest that the synaptosomal membrane fraction was probably enriched in NMDA receptors that are located within the synaptic membrane, as compared to the homogenate or light membranes, but may also have contained some receptors associated with post-synaptic vesicles.
In the frontal cortex, there was a greater decline in the expression of the NR2B subunit in the synaptic membrane than in the cell as a whole or the light membranes. This is consistent with the findings of a greater percent decline during aging in a study that used crude synaptosomes (synaptic membrane + vesicle-enriched compartment) (Magnusson et al., 2007) than in another study using whole homogenate (Magnusson et al., 2002) from C57BL/6 mice. We have previously reported significant declines in the mRNA expression of the NR2B subunit in the frontal cortex (Magnusson, 2000, 2001, Ontl et al., 2004), suggesting that some of the decline in NR2B subunit expression in frontal cortex is due to a loss of message. The present results indicate that there may be an additional effect of aging on NR2B subunit localization within the synaptic membranes of the frontal cortex. There are several mechanisms that affect normal expression of NR2B subunits within the synaptic membrane, including transportation of assembled receptors within vesicles to the vicinity of the synaptic membrane, insertion into the synaptic membrane or removal of the NR2B-containing receptors from the synaptic membrane for destruction or recycling (Carroll and Zukin, 2002, Prybylowski et al., 2005, Lau and Zukin, 2007). Aging may be acting on one or more of these. There might also be an increase in the density of NR2B subunits found within the extra-synaptic membrane (Tovar and Westbrook, 1999, Barria and Malinow, 2002, Thomas et al., 2006).
In the hippocampus, the effects of aging on protein expression of the NR2B subunit appeared to be similar between the synaptic membranes and the cells as a whole. This suggests that it was the same mechanism acting on both. Significant declines in mRNA expression of the NR2B subunit in the dentate gyrus of C57BL/6 mice (Magnusson, 2000) and a trend for a decline in the subunit protein in the light membrane fraction, indicate that the effect of aging on the NR2B subunit within the hippocampus might involve protein production. These results show that aging differentially affected the expression of the NR2B subunit in the frontal cortex as compared to the hippocampus.
Both regions showed a decrease in NR2B subunit expression within the synaptic membrane in the old mice. The NR2B subunit is normally found both in the synaptic and extra-synaptic membrane (Barria and Malinow, 2002, Prybylowski et al., 2005, Thomas et al., 2006). An age-related decline in the NR2B subunit within the synapse, with little or no change in the NR2A (Magnusson, 2000, Magnusson et al., 2002) and NR1 subunits could lead to a synaptic population of NMDA receptors that have decreased agonist affinity, faster kinetics, and reduced LTP associated with binding of calcium calmodulin kinase II (Kutsuwada et al., 1992, Monyer et al., 1992, Yamazaki et al., 1992, Ishii et al., 1993, Barria and Malinow, 2005) than in the young adult. If there was a shift to more extra-synaptic localization of NR2B subunits in the frontal cortex of aged animals, there could also be an increase in long-term depression (LTD) (Massey et al., 2004) and/or activation of a CREB shut-off pathway that interferes with induction of brain-derived neurotrophic factor (BDNF) and leads to a loss of mitochondrial membrane potential and cell death (Hardingham et al., 2002). Thus the aging changes seen in synaptic membrane expression of the NR2B subunit could be associated with changes in subunit composition and function of the synaptically-active NMDA receptors and/or a change in the interaction of NMDA receptors with other proteins involved in synaptic plasticity.
The present study showed some differences from our previous studies with respect to age-related changes in the NR2B subunit in the hippocampus and NR1 subunit in the frontal cortex (Magnusson et al., 2002, Magnusson et al., 2007). The hippocampal differences may be due to better dissection techniques used in the present study that increased the probability of extracting only hippocampus. The hippocampus was dissected out with the cortex, visualized, and then separated. The current hippocampal results are consistent with reports of significant declines in the protein expression of the NR2B subunit of Fisher 344 and F344BN rats within homogenates during aging (Eckles-Smith et al., 2000, Sonntag et al., 2000, Clayton et al., 2002b). Some of the differences between studies may be explained by the use of GAPDH as a loading control in the present study. Age-related changes in NR1 subunit protein were detected in mice in studies in which the subunit expression was not corrected for loading (Magnusson et al., 2002, Magnusson et al., 2007), but the subunit did not show aging changes when corrected for loading with syntaxin (Ontl et al., 2004). However, it should also be noted that variability in the effects of aging seems to be a characteristic of the NR1 subunit both within strains and between strains and species. Aged Fisher 344 rats show significant declines in NR1 subunit in the hippocampus (Eckles-Smith et al., 2000, Clayton et al., 2002b), but do not show any significant decline in the basal surface expression of this subunit (Clayton et al., 2002a). Long-Evans and F344BN rats do not show any significant changes in the NR1 subunit protein in the hippocampus during aging (Sonntag et al., 2000, Adams et al., 2001). This variability in NR1 subunit expression may be influenced by other factors during aging, such as behavioral experience (Das and Magnusson, 2008).
The middle-aged mice showed that good spatial memory was associated with higher expressions of the NMDA receptor subunits in the synaptic membrane of the hippocampus. This fits with the importance of hippocampal NMDA receptors in acquisition of spatial memory shown in younger animals (Morris, 1989, Heale and Harley, 1990, Steele and Morris, 1999). In contrast, within the group of old mice, the highest levels of expression of both NR2B and NR1 subunits in the hippocampal synaptic membrane were found in the aged mice with the poorest spatial memory. The range of NMDA receptor subunit expressions was similar between the old and middle-aged mice, so the negative relationship with memory in the old mice was not simply due to the subunit expression falling below a certain threshold. This suggests that a change occurred in the functioning of NMDA receptors in the synaptic membrane between 11 and 26 months of age in C57BL/6 mice. This could be due to compensatory changes induced by the decline in NMDA receptor expression that occurred at later ages than 11 months and/or aging changes that affect other molecules that interact with NMDA receptors.
There was also an association of high NR1 subunit expression in the hippocampal homogenate and poor performance in the place training trials in the old mice, although the opposite relationship was not seen with the younger mice. It is not clear why there should be separate relationships between whole cell expression and place trials and synaptic membrane expression and probe trials. A majority of studies show no role for hippocampal NMDA receptors in retrieval of spatial memories (Morris, 1989, Heale and Harley, 1990, Riekkinen and Riekkinen Jr., 1997, Steele and Morris, 1999), so both trials should reflect NMDA receptor involvement during acquisition of the memory. It is possible that extra-synaptic NMDA receptors, which should be present primarily in the homogenate, may play more of a role in place trial performance than in probe trials.
There is other evidence of a change in the role of NMDA receptors in aged animals. High densities of NMDA receptor binding within old rats in regions of the hippocampus have been shown to be associated with poor long-term memory retention in the water maze (Topic et al., 2007) and in dorsomedial striatium are related to poor attentional set shifting (Nicolle and Baxter, 2003). Aged rats that were unimpaired in a spatial memory task showed greater age-related declines in MK801 binding in the cortex and hippocampus than the impaired ones (Le Jeune et al., 1996). NMDA receptor antagonists, including mementine, a therapeutic used for Alzheimer’s disease; improve memory (Norris and Foster, 1999, Danysz and Parsons, 2003, Dias et al., 2007, Pieta Dias et al., 2007, Beracochea et al., 2008) and increase neurogenesis (Nacher et al., 2003) in aged individuals. Some evidence suggests that the blockade of the NMDA receptor may be protecting against excitotoxicity and oxidative damage (Pieta Dias et al., 2007). There is an increased responsiveness in aged animals to NMDA receptor-dependent low frequency stimulations leading to LTD, as compared to young (Norris et al., 1996, Huang and Kandel, 2006). Non-NMDA receptor-dependent LTP and LTD are more associated with good memory in aged rats than NMDA receptor-dependent (Lee et al., 2005, Boric et al., 2008). It has been suggested that the decline in NMDA receptor contribution to plasticity in aged animals may prevent the overwriting of old information (Yang et al., 2008). All of these studies suggest that the NMDA receptors present in aged animals function differently from those in young. It remains to be seen whether this could or should be reversed or prevented.
In conclusion, there were differences in the effects of aging on expression of the NR2B subunit within frontal cortex versus the hippocampus. In the frontal cortex there appeared to be an effect of aging on the localization of the NR2B subunit of the NMDA receptor to the synaptic membrane that was greater than changes in whole cell expression. The changes in synaptic expression of the NR2B subunit in the hippocampus appeared to be more related to declines throughout the cell, suggesting a problem with production. In the hippocampus, there was also a switch from high expression of the NR1 and NR2B subunits being associated with good memory in middle-aged mice to being related to poor memory in the aged mice. These data suggest that NMDA receptor functions, receptor subunit composition, and/or the environment in which the receptor interacted were not the same in the old mice as in younger mice and this may have contributed to the memory declines seen during aging. It is unclear at this time whether or not preventing age-related declines in NMDA receptor expression would be beneficial to memory in aged individuals.
Acknowledgments
This work was supported by NIH grant AG16322 to KRM. We would also like to thank David G. Standaert for his advice on the subfractionation protocol.
Abbreviations
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CO2
carbon dioxide
- CPP
[(±)-2-carboxypiperazin-4-yl] propyl-1-phosphonic acid
- CREB
cAMP response element binding protein
- EDTA
ethylenediamine tetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- H
homogenate
- HCl
hydrochloric acid
- IR
infra-red
- kDa
kilodalton
- LP1
lysis pellet 1 = synaptosomal membrane fraction
- LTD
long-term depression
- LTP
long-term potentiation
- mRNA
messenger ribonucleic acid
- N
north
- NE
northeast
- NMDA
N-methyl-D-aspartate
- NR1 subunit
mouse ζ1 subunit
- NR2A subunit
mouse ε1 subunit
- NR2B subunit
mouse ε2 subunit
- P.I
protease inhibitors
- P1
first pellet
- P2
second pellet = crude synaptosomal fraction
- P3
third pellet = light membrane fraction
- PSD-95
post-synaptic density protein, 95 kDa
- S1
first supernatant
- S2
second supernatant
- SDS
Sodium dodecyl sulfate
- SE
southeast
- SEM
standard error of the mean
- TBS
Tris buffered saline
- TBS-T
Tris buffered saline + Tween 20
- TE buffer
Tris HCl, EDTA and EGTA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Albert MS, Funkenstein HH. The effects of age: Normal variation and its relation to disease. In: Asbury AK, et al., editors. Diseases of the Nervous System: Clinical Neurobiology. Philadelphia: Saunders; 1992. pp. 598–611. [Google Scholar]
- Alessandri B, Battig K, Welzl H. Effects of ketamine on tunnel maze and water maze performance in the rat. Behav Neural Biol. 1989;52:194–212. doi: 10.1016/s0163-1047(89)90313-0. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. NMDA receptors and developmental plasticity in visual neocortex. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. Oxford: Oxford University Press; 1994. pp. 313–339. [Google Scholar]
- Barinaga M. Secrets of secretion revealed. Science. 1993;260:487–489. doi: 10.1126/science.8475382. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bashir Z, Alford S, Davies S, Randall A, Collingridge G. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Boucard A, Trocme-Thibierge C, Morain P. Improvement of contextual memory by S 24795 in aged mice: comparison with memantine. Psychopharmacology (Berl) 2008;196:555–564. doi: 10.1007/s00213-007-0987-5. [DOI] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: Effects on performance of a hippocampal-dependent task. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Boric K, Munoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J Neurosci. 2008;28:8034–8039. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhot M-C, Wolff M, Savova M, Malleret G, Hen R, Segu L. Protective effect of 5-HT1B receptor gene deletion on the age-related decline in spatial learning abilities in mice. Behav Brain Res. 2003;142:135–142. doi: 10.1016/s0166-4328(02)00400-x. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle JM, Mouton PR, Ingram DK, Jucker M. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging. 1998;19:599–606. doi: 10.1016/s0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older animals. Psychol Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Grosshans DR, Browning MD. Aging and surface expression of hippocampal NMDA receptors. J Biol Chem. 2002a;277:14367–14369. doi: 10.1074/jbc.C200074200. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002b;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely KN, Boehnke M. So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. Am J Hum Genet. 2007;81:1158–1168. doi: 10.1086/522036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18:S23–32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- Das SR, Magnusson KR. Relationship between mRNA expression of splice forms of the zeta1 subunit of the N-methyl-d-aspartate receptor and spatial memory in aged mice. Brain Res. 2008;1207:142–154. doi: 10.1016/j.brainres.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Dias C, Martins de Lima M, Presti-Torres J, Dornelles A, Garcia V, Scalco F, Guimaraes M, Castantino L, Budni P, Dal-Pizzol F, Schroder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146:1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Mol Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Evans GWP, Brennan PLM, Skorpanich MAM, Held DB. Cognitive mapping and elderly adults: Verbal and location memory for urban landmarks. J Gerontol. 1984;39:452–457. doi: 10.1093/geronj/39.4.452. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive funciton and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Frick KM, Burlingame LA, Arter JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan J-Y, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F, Dunnett S, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Nicolle MM. Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Genz A, Bretz F, Hothorn T. T.mvtnorm: Multivariate normal and t distribution. [accessed November, 2007];R package version 081. http://cran.r-project.org/doc/packages/mvtnorm.pdf. [Google Scholar]
- Gonzales RA, Brown LM, Jones TW, Trent RD, Westbrook SL, Leslie SW. N-methyl-D-aspartate mediated responses decrease with age in Fischer 344 rat brain. Neurobiol Aging. 1991;12:219–225. doi: 10.1016/0197-4580(91)90100-x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Zhou Y, Elder G, Li C, Lazzarini RA. Age-dependent spatial memory deficits in transgenic mice expressing the human mid-sized neurofilament gene: I. Mol Brain Res. 1996;42:62–70. doi: 10.1016/s0169-328x(96)00114-3. [DOI] [PubMed] [Google Scholar]
- Harris EW, Ganong A, Cotman CW. Long-term potentiation in the hippocampus involves activation in N-methyl-D-aspartate receptors. Brain Res. 1984;323:132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Head E, Mehta R, Hartley J, Kameka M, Cummings BJ, Cotman CW, Ruehl WW, Milgram NW. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- Heale V, Harley C. MK801 and AP5 impair acquisition, but not retention, of the Morris milk maze. Pharmacol Biochem Behav. 1990;36:145–149. doi: 10.1016/0091-3057(90)90140-d. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Huang YY, Kandel ER. Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus. Learn Mem. 2006;13:298–306. doi: 10.1101/lm.166906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. Cloning and expression of the epsilon4 subunit of the NMDA receptor channel. FEBS Lett. 1992;313:34–38. doi: 10.1016/0014-5793(92)81178-o. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3:732–737. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- Kirasic KC, Bernicki MR. Acquisition of spatial knowledge under conditions of temporospatial discontunity in young and elderly adults. Psychol Res. 1990;52:76–79. doi: 10.1007/BF00867215. [DOI] [PubMed] [Google Scholar]
- Kito S, Miyoshi R, Nomoto T. Influence of age on NMDA receptor complex in rat brain studied in in vitro autoradiography. J Histochem Cytochem. 1990;38:1725–1731. doi: 10.1177/38.12.2147708. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Le Jeune H, Cecyre D, Rowe W, Meaney MJ, Quirion R. Ionotropic glutamate receptor subtypes in the aged memory-impaired and unimpaired Long-Evans rat. Neuroscience. 1996;74:349–363. doi: 10.1016/0306-4522(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the most sensitive measure of water maze probe test performance? Front Integrat Neurosci. 2009;3:1–9. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Differential effects of aging on binding sites of the activated NMDA receptor complex in mice. Mech Ageing Dev. 1995;84:227–243. doi: 10.1016/0047-6374(95)01658-9. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: Correlations between receptor binding and spatial memory performance in C57Bl mice. Mech Ageing Dev. 1998a;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. The aging of the NMDA receptor complex. Front Biosci. 1998b;3:e70–80. doi: 10.2741/a368. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiol Aging. 2001;22:613–627. doi: 10.1016/s0197-4580(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scanga C, Wagner AE, Dunlop C. Changes in anesthetic sensitivity and glutamate receptors in the aging canine brain. J Gerontol: Biol Sci. 2000;55:B448–B454. doi: 10.1093/gerona/55.9.b448. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Aniya J, Wright KC, Ontl T, Xing Y, Bai L. Age-related deficits in mice preforming working memory tasks in a water maze. Behav Neurosci. 2003;117:485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 2007;8:43. doi: 10.1186/1471-2202-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumnainshi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- Mondadori C, Weiskrantz L, Buerki H, Petschke F, Fagg GE. NMDA receptor antagonists can enhance or impair learning performance in animals. Exp Brain Res. 1989;75:449–456. doi: 10.1007/BF00249896. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moore TE, Richards B, Hood J. Aging and the coding of spatial information. J Gerontol. 1984;39:210–212. doi: 10.1093/geronj/39.2.210. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Synaptic plasticity and learning: Selective impairment of learning in rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Davis M. The role of NMDA receptors in learning and memory. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. Oxford: Oxford University Press; 1994. pp. 340–375. [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Baxter MG. Glutamate receptor binding in the frontal cortex and dorsal striatum of aged rats with impaired attentional set-shifting. Eur J Neurosci. 2003;18:3335–3342. doi: 10.1111/j.1460-9568.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- Norris CM, Foster TC. MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol Learn Mem. 1999;71:194–206. doi: 10.1006/nlme.1998.3864. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontl T, Xing Y, Bai L, Kennedy E, Nelson S, Wakeman M, Magnusson KR. Development and aging of N-methyl-D-aspartate receptor expression in the prefrontal/frontal cortex of mice. Neuroscience. 2004;123:467–479. doi: 10.1016/j.neuroscience.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Beatty G, Gallagher M. Hippocampal 3H-CPP binding and spatial learning deficits in aged rats. Psychobiology. 1990;18:298–304. [Google Scholar]
- Pelleymounter MA, Smith M, Gallagher M. Spatial learning impairments in aged rats trained with a salient configuration of stimuli. Psychobiology. 1987;15:248–254. [Google Scholar]
- Pieta Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, Siciliani Scalco F, Rewsaat Guimaraes M, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146:1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Perry EK, Perry RH, Court JA. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 1992;588:277–286. doi: 10.1016/0006-8993(92)91586-4. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Fedele E, Risiglione C, Raiteri M. Age-related decrease of the NMDA receptor-mediated noradrenaline release in rat hippocampus and partial restoration by D-cycloserine. Eur J Pharm. 1993;231:129–134. doi: 10.1016/0014-2999(93)90693-c. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp P, Rosenberg R, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Riekkinen P., Jr Dorsal hippocampal muscarinic acteylcholine and NMDA receptors disrupt water maze navigation. NeuroReport. 1997;8:645–648. doi: 10.1097/00001756-199702100-00013. [DOI] [PubMed] [Google Scholar]
- Sharps MJ, Gollin ES. Memory for object locations in young and elderly animals. J Gerontol. 1987;42:336–341. doi: 10.1093/geronj/42.3.336. [DOI] [PubMed] [Google Scholar]
- Sierralta J, Mendoza C. PDZ-containing proteins: alternative splicing as a source of functional diversity. Brain Res Brain Res Rev. 2004;47:105–115. doi: 10.1016/j.brainresrev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-asparate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RGM. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tamaru M, Yoneda Y, Ogita K, Shimizu J, Nagata Y. Age-related decreases of the N-methyl-D-aspartate receptor complex in the rat cerebral cortex and hippocampus. Brain Res. 1991;542:83–90. doi: 10.1016/0006-8993(91)91001-h. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Topic B, Willuhn I, Palomero-Gallagher N, Zilles K, Huston JP, Hasenohrl RU. Impaired maze performance in aged rats is accompanied by increased density of NMDA, 5-HT1A, and alpha-adrenoceptor binding in hippocampus. Hippocampus. 2007;17:68–77. doi: 10.1002/hipo.20246. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol Aging. 1991;12:93–98. doi: 10.1016/0197-4580(91)90047-n. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992;300:39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- Yang Z, Krause M, Rao G, McNaughton BL, Barnes CA. Synaptic commitment: developmentally regulated reciprocal changes in hippocampal granule cell NMDA and AMPA receptors over the lifespan. J Neurophysiol. 2008;99:2760–2768. doi: 10.1152/jn.01276.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]
- Zyzak DR, Otto T, Eichenbaum H, Gallagher M. Cognitive decline associated with normal aging in rats: A neuropsychological approach. Learn Mem. 1995;2:1–16. doi: 10.1101/lm.2.1.1. [DOI] [PubMed] [Google Scholar]