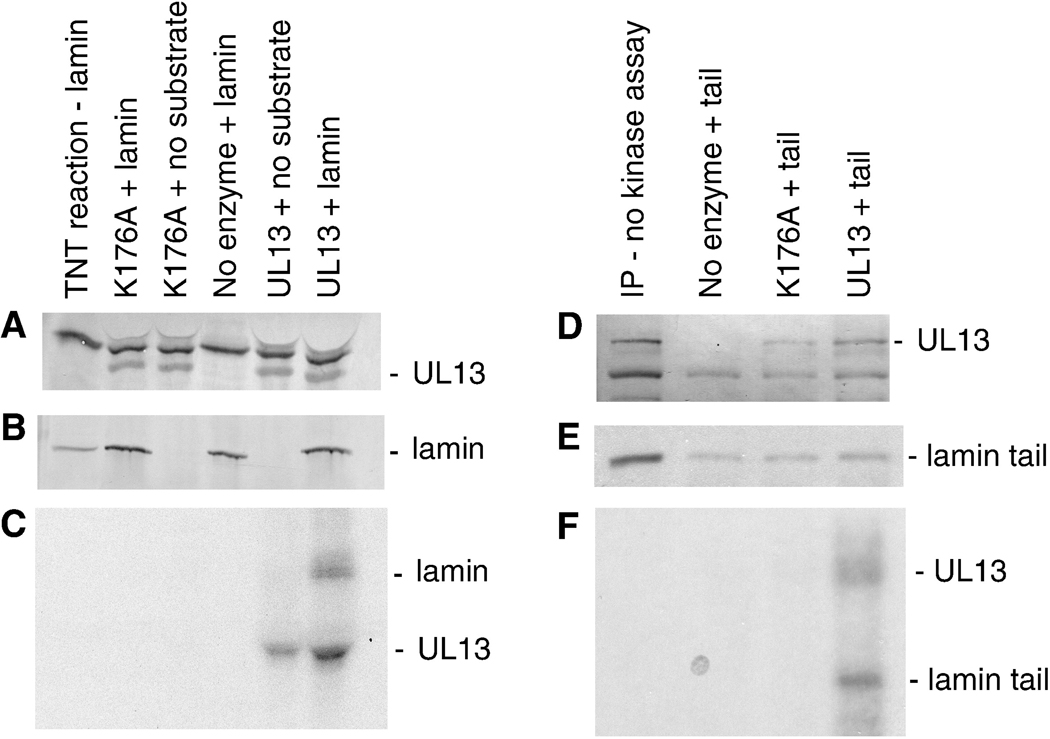
Figure 5. HSV-2 UL13-HA can directly phosphorylate the tail domain of lamin A.
In vitro transcribed and translated UL13-HA and UL13-K176A-HA, and full-length or tail domain of lamin A were isolated by mixed bed immunoprecipitation and the immunoprecipitates were subjected to an in vitro kinase assay. The products were resolved by SDS-PAGE (8% gel for full-length lamin and 12% gel for the tail domain), and detected by western blot and autoradiography. A) UL13-HA and UL13-K176A-HA detected with anti-HA antibody, B) full-length lamin A detected by western blot with anti-lamin antibody, and C) 32P-labeled proteins detected by autoradiography; D) UL13-HA and UL13-K176A-HA detected by western blot, E) 35S-labeled and 32P-labeled proteins and F) 32P-labeled proteins detected by autoradiography. The lamin tail domain was not discernible by western blot because it has the same mobility as light chain and other cross reacting bands.