Abstract
Introduction
We evaluated the feasibility of using an electronic protocol developed for research use (Research-eProtocol-insulin) for blood glucose management in usual intensive care unit clinical practice.
Methods
We implemented the rules of Research-eProtocol-insulin in the electronic medical record of the Intermountain Healthcare hospital system (Clinical-eProtocol-insulin) for use in usual clinical practice. We evaluated the performance of Clinical-eProtocol-insulin rules in the intensive care units of seven Intermountain Healthcare hospitals and compared this performance with the performance of Research-eProtocol-insulin at the LDS Hospital Shock/Trauma/Respiratory Intensive Care Unit.
Results
Clinician (nurse or physician) compliance with computerized protocol recommendations was 95% (of 21,325 recommendations) with Research-eProtocol-insulin and 92% (of 109,458 recommendations) with Clinical-eProtocol-insulin. The blood glucose distribution in clinical practice (Clinical-eProtocol-insulin) was similar to the research use distribution (Research-eProtocol-insulin); however, the mean values (119 mg/dl vs 113 mg/dl) were statistically different (P = 0.0001). Hypoglycemia rates in the research and practice settings did not differ: the percentage of measurements ≤40 mg/dl (0.11% vs 0.1%, P = 0.65) and the percentage of patients with at least one blood glucose ≤40 mg/dl (4.2% vs 3%, P = 0.23) were not statistically significantly different.
Conclusion
Our electronic blood glucose protocol enabled translation of a research decision-support tool (Research-eProtocol-insulin) to usual clinical practice (Clinical-eProtocol-insulin).
Keywords: clinical, clinical protocol, computer protocol, glucose, intensive care, replicability
Introduction
Clinical research results and clinical practice do not converge in many domains of health care.1,2 Uncertain medical research results and the absence of tools that enable easy translation of research results contribute to the gap between research-based evidence and clinical practice. Replicability is a basic requirement of science.1–9 For both clinical experiments and clinical care, replicability commonly requires detailed and adequately explicit methods.3,10–14 Unfortunately, many clinical trial experimental methods and most clinical care methods are not adequately explicit.15 A protocol rule such as “If blood glucose approaches the normal range, adjust with increments/decrements of 0.1–0.5 IU per hour”15,16 requires bedside clinician judgment that becomes part of the method. Because the reasons for clinician judgments are not known, and because these judgments frequently differ within and among clinicians, such methods that incorporate clinician judgment cannot be completely reported and therefore cannot be replicated.
Electronic protocols with adequate detail can be replicable clinical research and clinical care methods.17 We developed an electronic research protocol (Research-eProtocol-insulin) for the management of blood glucose with intravenous insulin for intensive care unit (ICU) patients.18 We tested the feasibility of using eProtocol-insulin to translate research results into clinical practice.
Methods
LDS Hospital Shock/Trauma/Respiratory Intensive Care Unit investigators iteratively developed and refined a point-of-care computerized protocol for clinical quality improvement.17,18 This computerized protocol was further refined in a formal, institutional review board-approved, multicenter research effort (Research-eProtocol-insulin; see Appendix in Thompson et al.19). Bedside clinicians (nurses or physicians) entered a blood glucose value in a simple single bedside computer screen (Figure 1). Research-eProtocol-insulin then generated one or more individualized patient recommendations. The most recent blood glucose determined the initial insulin infusion rate. The current infusion rate, the difference between the most recent glucose and the blood glucose target, and the rate of change of blood glucose determined subsequent insulin infusion rates. Research-eProtocol-insulin displayed the time remaining to the next mandated blood glucose measurement. Clinical imperatives could lead to earlier blood glucose measurements. The Shock/Trauma/Respiratory Intensive Care Unit had previously adopted an 80- to 110-mg/dl (4.4–6.1 mmol/liter) blood glucose target for their patients.15,16,20 This intensive care unit functioned as our refinement laboratory in an iterative refinement process. It was able to execute this role because of committed physician and nursing oversight, and an integrated electronic medical record that allowed the physician-investigator to aggregate data in real time from multiple patients.21,22 Independent applications of Research-eProtocol-insulin were installed at each bedside computer terminal. We supported as many as 12 patients simultaneously with Research-eProtocol-insulin.
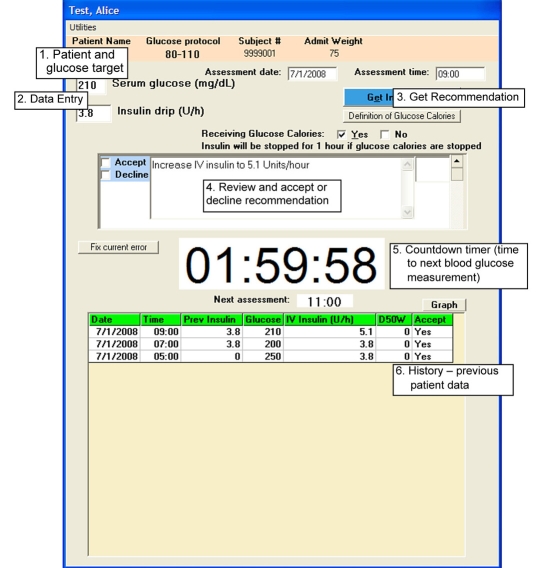
Figure 1.
Single bedside Research-eProtocol-insulin computer screen. 1. Patient name and blood glucose protocol target range. 2. Data entry fields. 3. Get eProtocol-insulin recommendation button (new intravenous insulin infusion rate). 4. New insulin infusion rate recommendation with Accept or Decline checkboxes to the left (white background becomes gray after bedside clinician accepts or declines the recommendation). 5. Countdown timer displays 1 hour 59 minutes and 58 seconds to the next eProtocol-insulin-recommended blood glucose measurement (at 11:00 hours—displayed in the Next assessment window below the countdown timer). 6. Previous patient data with dates and times (this can be toggled to a graphical display with the Graph button above and to the right of the table).
Arterial, venous, or finger-stick blood samples were subjected to blood glucose measurement at the bedside with a glucometer (LifeScan®) or in the hospital laboratory. Most measurements were bedside measurements of arterial blood. The protocol blood glucose target was 80–110 mg/dl. Research-eProtocol-insulin bedside individualized patient recommendations were evaluated and accepted or declined by bedside clinicians. If declined, the insulin infusion rate was set according to clinician judgment and was recorded in the Research-eProtocol-insulin database with the reason for declining the recommendation. If the clinician accepted the recommendation, the clinician was said to be compliant with the recommendation.
Once validated at LDS Hospital, Research-eProtocol-insulin rules were translated to a different platform and imbedded in the HELP2 Intermountain Healthcare electronic medical record system using a Java rules engine called Foresight.23 This translation was necessary because Research-eProtocol-insulin was developed on a platform designed for rapid prototyping and rules development at LDS Hospital. With this platform protocol rules can be created, visualized, and expressed in common medical terms and concepts (e.g., the patient is hyperglycemic). This is advantageous when meeting with clinicians to identify their decision-making processes and for explanation of Research-eProtocol-insulin outputs.24
The rapid prototyping platform is not integrated with HELP2 and is not available throughout Intermountain Healthcare. We translated Research-eProtocol-insulin manually. Each rule was rebuilt because of three major incompatibilities between the prototyping platform and Foresight: data model, terminology, and knowledge (rule) representation. Once translated, tested, and validated both in silico and clinically at the LDS Hospital, the clinical Foresight version of the protocol became available to all Intermountain inpatient facilities where HELP2 is installed (Clinical-eProtocol-insulin). Clinical-eProtocol-insulin was distributed to Intermountain Healthcare intensive care units through its Intensive Medical Clinical Program. The distribution was accompanied by an educational program in each ICU and a clinician support team available to answer questions about the operation of the computer and the rationale for the program's insulin decisions. A member of the implementation team reviewed reports daily for problems (including hypo-glycemia) in each hospital and pursued these problems with local clinical care providers.25 All patients supported at any time with Clinical-eProtocol-insulin were included in the data analysis. We used Shock/Trauma/Respiratory Intensive Care Unit data from patients previously supported with paper-based protocols with two different blood glucose targets (120–180 and 80–120 mg/dl) to calculate the mean interval between blood glucose measurements with paper-based protocols.
We used a generalized linear model to account for the patient effect and assessed statistical significance of differences of glucose values between the development (Shock/Trauma/Respiratory Intensive Care Unit at LDS Hospital) unit and the clinical practice units.26 All patients who were at any time treated with either version of eProtocol-insulin were included in the statistical analysis. The Intermountain Healthcare institutional review board approved publication of these quality improvement data.
Results
The mean interval between blood glucose measurements from previous work (during initial development of a computer protocol) in the Shock/Trauma/Respiratory Intensive Care Unit with paper-based protocols was 1.6 hours for the 120- to 180-mg/dl blood glucose target and 1.4 hours for the 80- to 120-mg/dl target.
Research-eProtocol-insulin generated 21,325 recommendations in 493 Shock/Trauma/Respiratory Intensive Care Unit patients who were supported by Research eProtocol-insulin for 4.1 ± 4.4 days. The mean blood glucose was 113 mg/dl [standard deviation (SD) 41], the median was 105 mg/dl, and 46% of measurements were within the 80- to 110-mg/dl target. We encountered blood glucose measurements ≤40 mg/dl in 0.1% of measurements and at least one blood glucose measurement ≤40 mg/dl in 3% of patients. Clinician compliance (acceptance) with Research-eProtocol-insulin recommendations was 95%. The mean ± SD time between blood glucose measurements with Research-eProtocol-insulin was 2.3 ± 1.1 hours (equivalent to 10.4 measurements/patient/day).
Thirteen Intermountain Healthcare intensive care units in seven hospitals used Clinical-eProtocol-insulin for 4.1 ± 6.1 days per patient (mean ± SD). The hospitals that housed these intensive care units varied from a 72-bed primary care hospital to a 480-bed tertiary care research and training hospital. Intensive care unit critical care physician oversight varied from intermittent part-time to full-time (24 hours/day, 7 days/week) coverage. Clinical-eProtocol-insulin generated 109,458 recommendations in 2296 patients receiving usual care management of blood glucose with intravenous insulin. These data were obtained from the raw, unfiltered, clinical database. The mean blood glucose for the patients using Clinical-eProtocol-insulin was 119 mg/dl (SD 48). The median was 108 mg/dl and 42% of measurements were within target 80–110 mg/dl. The mean blood glucose values and the percentage of measurements within target among the seven intensive care units using Clinical-eProtocol-insulin were statistically significantly different (P ≤ 0.001). We encountered blood glucose measurements ≤40 mg/dl in 0.11% of measurements and at least one blood glucose measurement ≤40 mg/dl in 4.2% of patients. Percentage measurements ≤40 mg/dl (0.1% vs 0.11%, P = 0.65) and the percentage of patients with at least one blood glucose ≤40 mg/dl (3% vs 4.2%, P = 0.23) were not statistically significantly different from the rates in the Shock/Trauma/Respiratory Intensive Care Unit patients supported with Research-eProtocol-insulin. Clinician compliance (acceptance) with Clinical-eProtocol-insulin recommendations was 92%. The mean ±SD time between blood glucose measurements with Clinical-eProtocol-insulin was 2.1 ± 0.9 hours (equivalent to 11.4 measurements/patient/day).
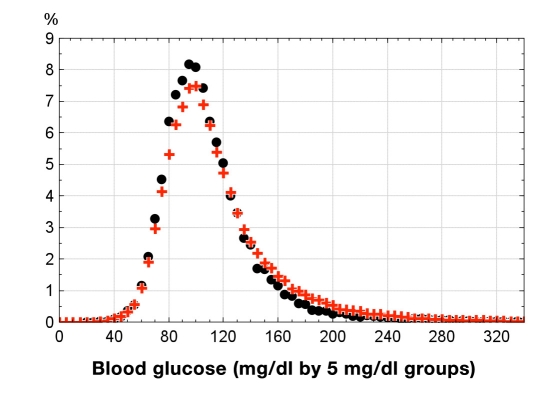
The Clinical-eProtocol-insulin blood glucose distribution was difficult to distinguish from Research-eProtocol-insulin in the Shock/Trauma/Respiratory Intensive Care Unit of the LDS hospital (Figure 2), although differences between hospitals were statistically significant. These statistically significant differences were much smaller than the differences in blood glucose distributions between institutions before using eProtocol-insulin.18 The mean blood glucose from the Shock/Trauma/Respiratory Intensive Care Unit with Research-eProtocol-insulin was statistically different from the corresponding values for aggregated data from the 13 Intermountain Healthcare intensive care units in 7 hospitals with Clinical-eProtocol-insulin (P < 0.0001). Percent measurements ≤40 mg/dl and the percentage of patients with at least one blood glucose ≤40 mg/dl with Research-eProtocol-insulin from the Shock/Trauma/Respiratory Intensive Care Unit were not statistically different from the corresponding values for aggregated data from the 13 Intermountain Healthcare intensive care units in seven hospitals that used Clinical-eProtocol-insulin (P = 0.65 and 0.23, respectively).
Figure 2.
Distribution of percent (%) of blood sugar measurements during usual clinical care use of eProtocol-insulin in 13 intensive care units of seven intermountain Healthcare Hospitals (+) and in the LDS Hospital Shock/Trauma/Respiratory Intensive Care Unit (•), the eProtocol-insulin research development site.
Discussion
Overall clinician compliance with protocol recommendations in clinical practice was high (92%) and exceeded those commonly reported for guideline adherence.17,27 The mean ± SD times between blood glucose measurements were similar with Research-eProtocol-insulin (2.3 ± 1.1 hours) and Clinical-eProtocol-insulin (2.1 ± 0.9 hours). These intervals were longer than those associated with paper-based protocol use (1.6 and 1.4 hours). The nurse burdens therefore appear similar in the research development and in the usual clinical care ICU sites and both appear to be less than those with previously used paper-based protocols to manage blood glucose.
The occurrence of a blood glucose measurement ≤40 mg/dl was infrequent, indicating adequate patient safety. The performance of Clinical-eProtocol-insulin in clinical practice was statistically different from the performance of Research-eProtocol-insulin in the research environment at the Shock/Trauma/Respiratory Intensive Care Unit, but we could not distinguish differences in blood glucose distributions we thought would likely be important clinically (Figure 2). We believe that these preliminary results establish the feasibility of using an electronic tool to link clinical research and usual clinical care in intensive care units. This use of an electronic tool is directly responsive to the need for methods to affect translation of research results to clinical practice.28,29 If widely applied in clinical practice, it could help bridge the gap between the evidence base of research study results and clinical practice application.30,31 In addition, because it is adequately explicit, use of eProtocol-insulin in a clinical trial should enable clinical experiments that are replicable and that produce more reliable results.17
eProtocol-insulin recommendations fall within the range of published algorithms (see Figure 3 in Thompson et al.19). Of the many guidelines and protocols produced to guide clinical management, most require clinicians to use judgment to make protocol decisions. Because different clinicians make decisions differently when faced with the same clinical state, these guidelines and protocols do not contain enough detail to elicit the same decision from different clinicians. They cannot, then, achieve replicability of decisions. In contrast, eProtocol-insulin is adaptive, replicable, and can deliver individualized therapy to patients.17,27 We believe eProtocol-insulin possesses an advantage because of its multicenter refinement and validation19 and because its results are replicable in diverse ICUs in quality improvement,18 research,19 and usual clinical care applications.
This was a quality improvement test of feasibility of exporting our research tool into clinical practice. We intentionally had no input or contact with the clinicians in practice but left the implementation strategy to the clinicians in charge of our intensive medical clinical program. We did not survey nurses or physicians and did not intrude on the clinical care setting to obtain any information because we did not want to influence their use, for clinical care purposes, of Clinical-eProtocol-insulin. We therefore have no readily available data that could be used to describe the patient subset receiving support with Clinical-eProtocol-insulin.
Clinical-eProtocol-insulin was implemented as a clinical care protocol. The responsible practicing physicians elected independently to use Clinical-eProtocol-insulin for some of their patients. Since this was a usual clinical care implementation, we neither captured the reasons for which practicing physicians elected to use Clinical-eProtocol-insulin, tracked hyperglycemic patients not managed with this protocol, nor surveyed nurses or physicians. We therefore have no data that could be used to describe the time spent by practicing clinicians in application of this protocol. To our knowledge the clinicians used no common selection criterion other than the judgment of the clinician to control blood glucose with intravenous insulin.
Limitations of this preliminary report include lack of knowledge of why only some patients were chosen for Clinical-eProtocol-insulin use. We do not know the attributes of this patient subset and how they compared with the majority of patients who did not receive care with Clinical-eProtocol-insulin. We asked only one question in this feasibility effort: can our research eProtocol-insulin be exported to the clinical care setting and used for usual clinical care? Our results indicate that it is feasible to export the Research-eProtocol-insulin decision-support tool to the clinical practice setting. We did not address the attributes of its use, its efficacy, effectiveness, cost/benefit ratio, burden on bedside clinicians, patient selection criteria, acceptance or participation by physicians, or related issues. These are all important issues that should be addressed in future studies.
The portability and exchange of decision rules are known problems within the clinical informatics community.32,33 Despite the availability of data and knowledge representation standards, low adoption has impeded efficient knowledge sharing, even inside the same institution.32,33 As a result, linking decision rules to electronic medical records requires significant effort and support from local institutional information technology departments. We believe such support would be difficult to obtain without additional external funding in many clinical environments. Until the existing standards are widely adopted, and interoperability is established between different electronic medical record systems, the transfer of decision rules between platforms will continue to require manual conversions. This article demonstrated that such manual conversions are possible and can produce comparable results in both research and clinical care environments.
Conclusion
eProtocol-insulin enabled translation of research results to clinical practice. eProtocol-insulin-associated blood glucose distributions in the research and clinical care settings were similar. Prospective studies of efficacy and of practicing clinicians' extent of adoption of eProtocol-insulin are needed.
Acknowledgments
The authors acknowledge with gratitude the critical comments and advice from J. Michael Dean, M.D. (University of Utah, Department of Pediatrics, Primary Children's Medical Center), and the statistical calculations provided by Hui Zhang, Ph.D. (Harvard University, Biostatistics Center, Massachusetts General Hospital). We are indebted to our many R.N. and M.D. clinician colleagues who enabled the completion of this work.
Abbreviations
- ICU
intensive care unit
- SD
standard deviation
Appendix
Reengineering Critical Care Clinical Research Investigators
The following persons and institutions are participants in the BAA Roadmap Initiative Reengineering Research in Critical Care:
Principal Investigator and Steering Committee Chair: A. Morris
Phase One Protocol Committee:
B. T. Thompson (Chair), K. H. Lee, J. Orme, A. Morris, P. Luckett, J. Truwit, D. Willson, D. Hite, R. Brower, G. Bernard
Clinical Coordinating Center:
B. T. Thompson, D. Schoenfeld, C. Oldmixon, H. Zheng, C. Bliss
Data and Safety Monitoring Board:
H. Wiedemann (Chair), G. Rubenfeld, M. Meade, S. Anand
Clinical Centers:
Primary Childrens Medical Center: E. Hirshberg, G. Larsen
Children's Hospital of Philadelphia: V. Nadkarni, V. Srinivasan, C. Bayer Roth, L. Hutchins
Vanderbilt University: G. B. Bernard, S. Bozeman
Wake Forest University: R. D. Hite, A. Howard
Massachusetts General Hospital: B. T. Thompson, C. Oldmixon
Johns Hopkins University: R. Brower, K. Boucher
LDS Hospital: J. Orme, L. Baumann
University of Virginia: J. Truwit, M. Marshall
University of Virginia Children's Hospital: D. Willson, M. Ball
Yale University: C. Bogue, V. Faustino, I. Lazar
Penn State Children's Hospital, Hershey: N. Thomas, J. Hess
Baystate Medical Center: J. Steingrub, M. Tidswell, L. Kozikowski
Vanderbilt Childrens: N. Patel, T. Shalaby
Childrens Hospital Central California: A. L. Graciano
Hospital for Sick Children: P. Cox, A. Guerguerian
St. Justine Hospital: J. Lacroix, G. Cannizzaro
Dartmouth Hitchcock Medical Center: D. Levin, D. Jarvis
Childrens Hospital Minnesota/St. Paul: Kurachek, L Blumberg
Childrens Hospital Michigan: S. Heidemann
Childrens Hospital Los Angeles: C. Newth, F. Fajardo
Children's Medical Center Dallas: P. Luckett
Baylor Childrens: L. Jefferson
Childrens Hospital Boston: A. Randolph
Funding
Funding was provided in part by the National Institutes of Health (#HHSN268200425210C), the Deseret Foundation, and Intermountain Health Care, Inc.
References
- 1.Sterman JD. Learning from evidence in a complex world. Am J Public Health. 2006;96(3):505–514. doi: 10.2105/AJPH.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28(1):413–433. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- 3.Campbell D, Stanley J. Experimental and quasi-experimental designs for research. Boston: Houghton Mifflin Co.; 1966. (reprinted from Handbook of Research on Teaching, 1963) [Google Scholar]
- 4.Babbie E. Survey research methods. Belmont, CA: Wadsworth Publishing Co.; 1990. [Google Scholar]
- 5.Guyatt G, Sackett D, Cook D. User's guide to the medical literature. II. How to use an article about therapy or prevention; A. Are the results of the study valid? JAMA. 1993;270(21):2598–2601. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- 6.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 8.Barrow JD. The book of nothing. New York: Pantheon Books; 2000. [Google Scholar]
- 9.Hawe P, Shiell A, Riley T. Complex interventions: how “out of control” can a randomised controlled trial be? BMJ. 2004;328(7455):1561–1563. doi: 10.1136/bmj.328.7455.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pocock SJ. Clinical trials: a practical approach. New York, NY: John Wiley & Sons; 1983. [Google Scholar]
- 11.Bailey RW. Human Performance engineering. 3rd ed. Upper Saddle River, NJ: Prentice Hall; 1996. [Google Scholar]
- 12.Piantadosi S. Clinical trials—a methodologic perspective. New York: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 13.Hulley S, Cummings S, Warren S, Grady D, Hearst N, Newman T. Designing Clinical Research. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 14.Sackett D, Haynes R, Guyatt G, Tugwell P. Clinical epidemiology: a basic science for clinical medicine. 2nd ed. Boston, MA: Little, Brown; 1991. [Google Scholar]
- 15.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 16.van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 17.Morris A. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med. 2000;132:373–383. doi: 10.7326/0003-4819-132-5-200003070-00007. [DOI] [PubMed] [Google Scholar]
- 18.Morris AH, Orme J, Jr, Truwit JD, Steingrub J, Grissom C, Lee KH, Li GL, Thompson BT, Brower R, Tidswell M, Bernard GR, Sorenson D, Sward K, Zheng H, Schoenfeld D, Warner H. A replicable method for blood glucose control in critically ill patients. Crit Care Med. 2008;36(6):1787–1795. doi: 10.1097/CCM.0b013e3181743a5a. [DOI] [PubMed] [Google Scholar]
- 19.Thompson BT, Orme JF, Zheng H, Luckett PM, Truwit JD, Willson DF, Hite RD, Brower RG, Bernard GR, Curley MA, J Steingrub JS, Sorenson DK, Sward K, Hirshberg E, Morris AH. Multicenter validation of a computer-based clinical decision support tool for glucose control in adult and pediatric intensive care units. J Diabetes Sci Technol. 2008;2(3):357–368. doi: 10.1177/193229680800200304. for the Reengineering Critical Care Clinical Research Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 21.Warner H, Olmsted C, Rutherford B. HELP—a program for medical decision-making. Comp Biomed Res. 1972;5(1):65–74. doi: 10.1016/0010-4809(72)90007-9. [DOI] [PubMed] [Google Scholar]
- 22.Kuperman GJ, Garder RM, Pryor TA. HELP: a dynamic hospital information system. New York: Springer-Verlag; 1991. [Google Scholar]
- 23.Rocha R, Bradshaw R, Hulse N, Rocha B. The clinical knowledge management infrastructure of intermountain healthcare. In: Greenes R, editor. Clinical decision support: the road ahead. Boston, MA: Academic Press; 2006. pp. 469–502. [Google Scholar]
- 24.Sorenson D, Grissom CK, Carpenter L, Austin A, Sward K, Napoli L, Warner HR, Morris AH Reengineering Clinical Research in Critical Care Investigators. A frame-based representation for a bedside ventilation weaning protocol. J Biomed Informatics. 2008;41(3):461–468. doi: 10.1016/j.jbi.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washington V, Orme J, Parker B, Anderson S, Holmen J, Nelson N, Lloyd J, Allen J, Jephson A, Sward K, Sorenson D, Clemmer T. Hypoglycemia factors and clinical sequelae with a computerized intravenous insulin glucose control protocol. Chest. 2006;130:A220. [Google Scholar]
- 26.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 27.Morris A. The importance of protocol-directed patient management for research on lung-protective ventilation. In: Dreyfuss D, Saumon G, Hubamyr R, editors. Ventilator-induced lung injury. New York: Taylor & Francis Group; 2006. pp. 537–610. [Google Scholar]
- 28.Westfall JM, Mold J, Fagnan L. Practice-based research–“Blue Highways” on the NIH roadmap. JAMA. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 29.Woolf SH, Johnson RE. The break-even point: when medical advances are less important than improving the fidelity with which they are delivered. Ann Family Med. 2005;3(6):545–552. doi: 10.1370/afm.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 31.Green LA, Fryer GE, Jr, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344(26):2021–2025. doi: 10.1056/NEJM200106283442611. [DOI] [PubMed] [Google Scholar]
- 32.Hripcsak G, Clayton PD, Pryor TA, Haug P, Wigertz OB, Van der lei J. The Arden sytnax for medical logic modules. Proceedings of the Fourteenth Annual Symposium on Computer Applications in Medical Care: IEEE Computer Soc Press; 1990. 200–4. [Google Scholar]
- 33.Hripsak G, Ludemann P, Pryor T, Wigertz O, Clayton P. Rationale for the Arden Syntax. Comput Biomed Res. 1994;27(4):291–324. doi: 10.1006/cbmr.1994.1023. [DOI] [PubMed] [Google Scholar]