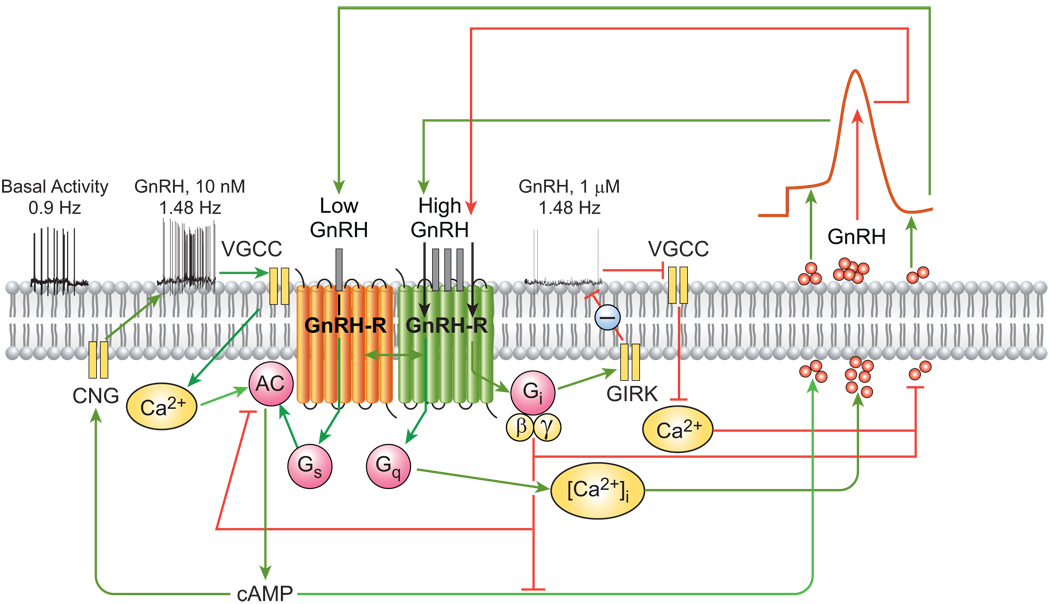
Figure 1.
Proposed mechanism of autocrine control of pulsatile GnRH secretion. (i) Firing of Ca2+-dependent action potentials in GnRH neurons promotes Ca2+ influx, activation of Ca2+-dependent adenylyl cyclase (AC), increased cAMP production, and elevated GnRH secretion. (ii) GnRH-induced stimulation of the endogenous GnRH-R expressed in GnRH neurons activates three G proteins, as indicated by the time- and dose-dependent release of their specific α-subunits from the plasma membrane. This is associated with increased inositol phosphate production and [Ca2+]i mobilization, as well as prominent increases in GnRH peak amplitude. Agonist activation of GnRH-Rs in GnRH neurons regulates AC activity in a biphasic manner, such that cAMP production is stimulated at nanomolar agonist levels and decreased by micromolar concentrations. (iii) Neuronal GnRH-Rs also interact with Gi proteins. The autocrine switch from Gs to Gi at high local GnRH concentrations interrupts the rise in neurosecretion and is followed by a fall to baseline and subsequent reactivation of secretion via the resurgent Ca2+/cAMP signaling pathways. VSCC, voltage-sensitive calcium channels; AC, adenylyl cyclase; CNG, cyclic nucleotide gated channels; GIRK, G protein-activated inwardly rectifying potassium channels. Green and red lines indicate stimulatory and inhibitory actions, respectively.