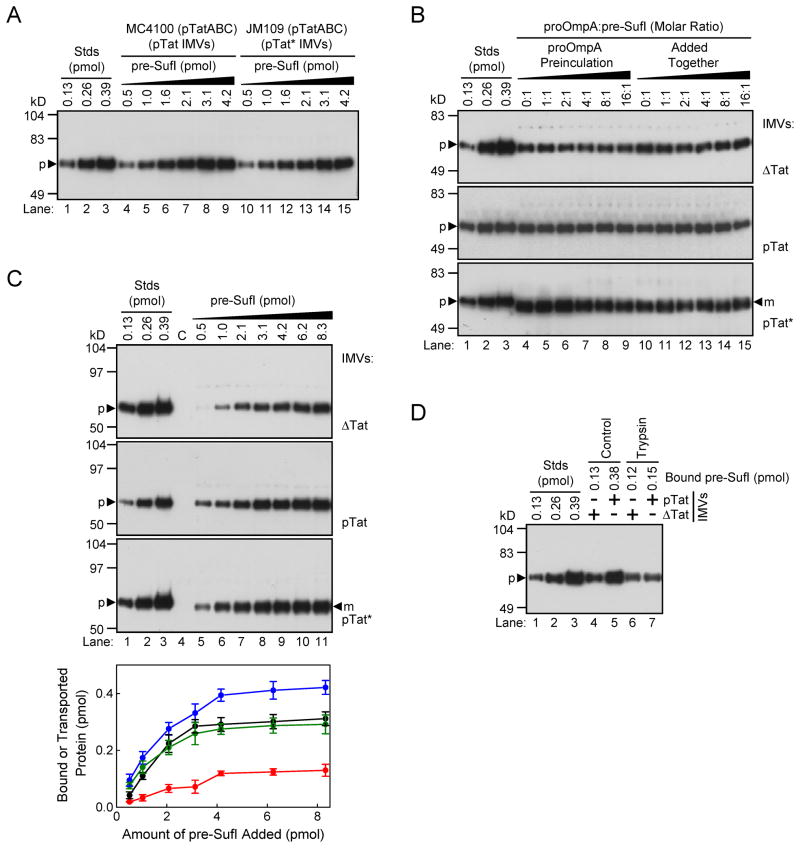
Figure 1. Quantification of precursor-lipid and precursor-translocon interactions.
All gels in this figure are anti-SufI immunoblots. (A) Membrane binding assay. The indicated amounts of pre-SufI were incubated with pTat or pTat* IMVs at pH 8.0 and then sedimented. The gel shows the amount of precursor protein recovered in the washed pellet fraction. Lanes 1–3 are quantification standards. (B) Competition between Sec and Tat precursors. Shown is the amount of membrane-bound (top and middle) and transported (bottom) pre-SufI in the presence of various molar equivalents of proOmpA-HisC. The proOmpA-HisC protein was preincubated with IMVs for 10 min prior to pre-SufI addition (lanes 4–9), or simultaneously with pre-SufI (lanes 10–15). Transport reactions were initiated with 4 mM NADH. Each lane contains the Sec chaperone SecB (25 μM). (C) Concentration dependence of membrane binding and transport. The binding of pre-SufI to ΔTat (top) and pTat (middle) IMVs was assayed as in A (pH 8.0). Transport into pTat* IMVs (bottom) was initiated by the addition of 4 mM NADH. Control lanes are devoid of pre-SufI for the binding assays, or with 8.3 pmol pre-SufI but no NADH for the transport assay. The plot shows the quantification of the pre-SufI bound to non-translocon components of the membrane (ΔTat IMVs, red), the pre-SufI bound to both Tat translocons and non-Tat membrane component (pTat IMVs, blue), and the pre-SufI transported into pTat* IMVs (black) (n = 3). The amount of pre-SufI bound to TatABC (green) was estimated by subtracting the red curve from the blue curve. (D) Binding to trypsin-treated IMVs. Membrane binding interactions were assayed as in A.