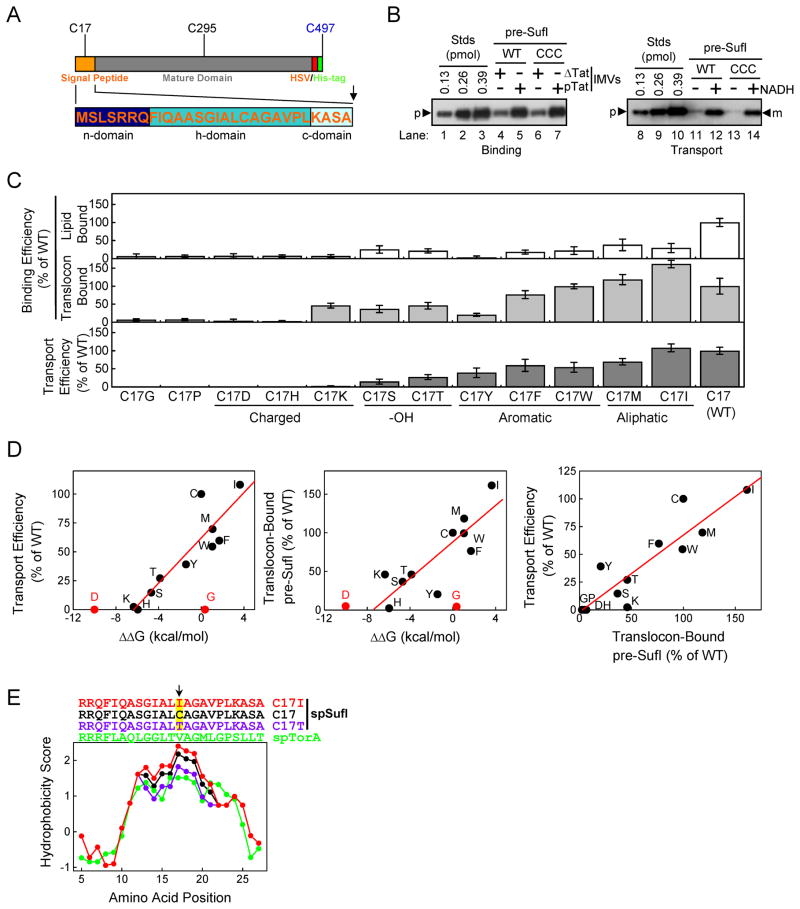
Figure 4. Effect of the hydrophobicity of the amino acid at position 17 in the pre-SufI signal peptide on membrane binding and transport efficiency.
(A) Domain structure of pre-SufI. C17 and C295 are endogenous cysteines and C497 was added for fluorescent dye labeling, yielding pre-SufI-CCC. (B) Membrane binding and transport efficiency of pre-SufI-CCC. These anti-SufI immunoblots show that addition of C497 has no effect on the membrane binding and transport efficiency of pre-SufI. (C) Membrane binding and transport efficiency of C17 mutants (n = 3). (D) Pairwise correlation between the ΔΔG (according to cyclohexane-to-water partition coefficients (Radzicka et al., 1988), the translocon binding efficiency and the transport efficiency for C17 mutants. For the left and middle plots, the data points for asparatic acid (D) and glycine (G) were not included in the best-fit line determination because they appear to be outliers. Once the transport efficiency reaches zero, a more negative ΔΔG is expected to have no effect, which explains the D point. (E) Kyte and Doolittle (Kyte & Doolittle, 1982) hydropathy plot for the signal peptides of pre-SufI (black), pre-SufI-ICC (red), pre-SufI-TCC (violet) and pre-TorA (green) using a scanning window of 9 residues.